Meropenem inhibits D,D-carboxypeptidase activity in Mycobacterium tuberculosis
- PMID: 22906310
- PMCID: PMC3468717
- DOI: 10.1111/j.1365-2958.2012.08199.x
Meropenem inhibits D,D-carboxypeptidase activity in Mycobacterium tuberculosis
Abstract
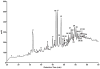
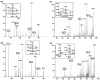
Carbapenems such as meropenem are being investigated for their potential therapeutic utility against highly drug-resistant tuberculosis. These β-lactams target the transpeptidases that introduce interpeptide cross-links into bacterial peptidoglycan thereby controlling rigidity of the bacterial envelope. Treatment of Mycobacterium tuberculosis (Mtb) with the β-lactamase inhibitor clavulanate together with meropenem resulted in rapid, polar, cell lysis releasing cytoplasmic contents. In Mtb it has been previously demonstrated that 3-3 cross-linkages [involving two diaminopimelate (DAP) molecules] predominate over 4-3 cross-linkages (involving one DAP and one D-alanine) in stationary-phase cells. We purified and analysed peptidoglycan from Mtb and found that 3-3 cross-linkages predominate throughout all growth phases and the ratio of 4-3/3-3 linkages does not vary significantly under any growth condition. Meropenem treatment was accompanied by a dramatic accumulation of unlinked pentapeptide stems with no change in the tetrapeptide pools, suggesting that meropenem inhibits both a D,D-carboxypeptidase and an L,D-transpeptidase. We purified a candidate D,D-carboxypeptidase DacB2 and showed that meropenem indeed directly inhibits this enzyme by forming a stable adduct at the enzyme active site. These results suggest that the rapid lysis of meropenem-treated cells is the result of synergistically inhibiting the transpeptidases that introduce 3,3-cross-links while simultaneously limiting the pool of available substrates available for cross-linking.
Published 2012. This article is a U.S. Government work and is in the public domain in the USA.
Figures
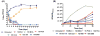



Similar articles
-
In vitro cross-linking of Mycobacterium tuberculosis peptidoglycan by L,D-transpeptidases and inactivation of these enzymes by carbapenems.Antimicrob Agents Chemother. 2013 Dec;57(12):5940-5. doi: 10.1128/AAC.01663-13. Epub 2013 Sep 16. Antimicrob Agents Chemother. 2013. PMID: 24041897 Free PMC article.
-
Structural basis for the inhibition of Mycobacterium tuberculosis L,D-transpeptidase by meropenem, a drug effective against extensively drug-resistant strains.Acta Crystallogr D Biol Crystallogr. 2013 Mar;69(Pt 3):420-31. doi: 10.1107/S0907444912048998. Epub 2013 Feb 16. Acta Crystallogr D Biol Crystallogr. 2013. PMID: 23519417 Free PMC article.
-
Inactivation of Mycobacterium tuberculosis l,d-transpeptidase LdtMt₁ by carbapenems and cephalosporins.Antimicrob Agents Chemother. 2012 Aug;56(8):4189-95. doi: 10.1128/AAC.00665-12. Epub 2012 May 21. Antimicrob Agents Chemother. 2012. PMID: 22615283 Free PMC article.
-
Imipenem-Relebactam and Meropenem-Vaborbactam: Two Novel Carbapenem-β-Lactamase Inhibitor Combinations.Drugs. 2018 Jan;78(1):65-98. doi: 10.1007/s40265-017-0851-9. Drugs. 2018. PMID: 29230684 Review.
-
Evolution of peptidoglycan biosynthesis under the selective pressure of antibiotics in Gram-positive bacteria.FEMS Microbiol Rev. 2008 Mar;32(2):386-408. doi: 10.1111/j.1574-6976.2007.00097.x. Epub 2008 Feb 11. FEMS Microbiol Rev. 2008. PMID: 18266857 Review.
Cited by
-
Peptidoglycan synthesis in Mycobacterium tuberculosis is organized into networks with varying drug susceptibility.Proc Natl Acad Sci U S A. 2015 Oct 20;112(42):13087-92. doi: 10.1073/pnas.1514135112. Epub 2015 Oct 5. Proc Natl Acad Sci U S A. 2015. PMID: 26438867 Free PMC article.
-
Proteomic characterization of Mycobacterium tuberculosis subjected to carbon starvation.mSystems. 2025 May 20;10(5):e0153024. doi: 10.1128/msystems.01530-24. Epub 2025 Apr 15. mSystems. 2025. PMID: 40231840 Free PMC article.
-
Exploring β-lactam interactions with DacB1: unraveling optimal therapies for combating drug-resistant Mycobacterium tuberculosis.mBio. 2025 Aug 13;16(8):e0137225. doi: 10.1128/mbio.01372-25. Epub 2025 Jul 10. mBio. 2025. PMID: 40637416 Free PMC article.
-
Mutation landscape of acquired cross-resistance to glycopeptide and β-lactam antibiotics in Enterococcus faecium.Antimicrob Agents Chemother. 2015 Sep;59(9):5306-15. doi: 10.1128/AAC.00634-15. Epub 2015 Jun 15. Antimicrob Agents Chemother. 2015. PMID: 26077262 Free PMC article.
-
Penicillin Binding Proteins and β-Lactamases of Mycobacterium tuberculosis: Reexamination of the Historical Paradigm.mSphere. 2022 Feb 23;7(1):e0003922. doi: 10.1128/msphere.00039-22. Epub 2022 Feb 23. mSphere. 2022. PMID: 35196121 Free PMC article.
References
-
- Arbeloa A, Hugonnet JE, Sentilhes AC, Josseaume N, Dubost L, Monsempes C, Blanot D, Brouard JP, Arthur M. Synthesis of mosaic peptidoglycan cross-bridges by hybrid peptidoglycan assembly pathways in gram-positive bacteria. J Biol Chem. 2004;279:41546–41556. - PubMed
-
- Atrih A, Foster SJ. The role of peptidoglycan structure and structural dynamics during endospore dormancy and germination. Antonie Van Leeuwenhoek. 1999;75:299–307. - PubMed
-
- Barry CE, 3rd, Lee RE, Mdluli K, Sampson AE, Schroeder BG, Slayden RA, Yuan Y. Mycolic acids: structure, biosynthesis and physiological functions. Prog Lipid Res. 1998;37:143–179. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

