The arginine-rich N-terminal domain of ROP18 is necessary for vacuole targeting and virulence of Toxoplasma gondii
- PMID: 22906355
- PMCID: PMC3683533
- DOI: 10.1111/cmi.12022
The arginine-rich N-terminal domain of ROP18 is necessary for vacuole targeting and virulence of Toxoplasma gondii
Abstract
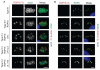
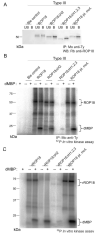
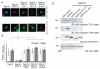
Toxoplasma gondii uses specialized secretory organelles called rhoptries to deliver virulence determinants into the host cell during parasite invasion. One such determinant called rhoptry protein 18 (ROP18) is a polymorphic serine/threonine kinase that phosphorylates host targets to modulate acute virulence. Following secretion into the host cell, ROP18 traffics to the parasitophorous vacuole membrane (PVM) where it is tethered to the cytosolic face of this host-pathogen interface. However, the functional consequences of PVM association are not known. In this report, we show that ROP18 mutants altered in an arginine-rich domain upstream of the kinase domain fail to associate to the PVM following secretion from rhoptries. During infection, host cells upregulate immunity-related GTPases that localize to and destroy the PVM surrounding the parasites. ROP18 disarms this host innate immune pathway by phosphorylating IRGs in a critical GTPase domain and preventing loading on the PVM. Vacuole-targeting mutants of ROP18 failed to phosphorylate Irga6 and were unable to divert IRGs from the PVM, despite retaining intrinsic kinase activity. As a consequence, these mutants were avirulent in a mouse model of acute toxoplasmosis. Thus, the association of ROP18 with the PVM, mediated by its N-terminal arginine-rich domain, is critical to its function as a virulence determinant.
© 2012 Blackwell Publishing Ltd.
Figures



Similar articles
-
Secretion of Rhoptry and Dense Granule Effector Proteins by Nonreplicating Toxoplasma gondii Uracil Auxotrophs Controls the Development of Antitumor Immunity.PLoS Genet. 2016 Jul 22;12(7):e1006189. doi: 10.1371/journal.pgen.1006189. eCollection 2016 Jul. PLoS Genet. 2016. PMID: 27447180 Free PMC article.
-
Toxoplasma gondii GRA60 is an effector protein that modulates host cell autonomous immunity and contributes to virulence.Cell Microbiol. 2021 Feb;23(2):e13278. doi: 10.1111/cmi.13278. Epub 2020 Oct 23. Cell Microbiol. 2021. PMID: 33040458
-
Toxoplasma IWS1 Determines Fitness in Interferon-γ-Activated Host Cells and Mice by Indirectly Regulating ROP18 mRNA Expression.mBio. 2023 Feb 28;14(1):e0325622. doi: 10.1128/mbio.03256-22. Epub 2023 Jan 30. mBio. 2023. PMID: 36715543 Free PMC article.
-
The secreted kinase ROP18 defends Toxoplasma's border.Bioessays. 2011 Sep;33(9):693-700. doi: 10.1002/bies.201100054. Epub 2011 Jul 20. Bioessays. 2011. PMID: 21773979 Free PMC article. Review.
-
Biogenesis of and activities at the Toxoplasma gondii parasitophorous vacuole membrane.Subcell Biochem. 2008;47:155-64. doi: 10.1007/978-0-387-78267-6_12. Subcell Biochem. 2008. PMID: 18512349 Review.
Cited by
-
Strain-specific disruption of interferon-stimulated N-myc and STAT interactor (NMI) function by Toxoplasma gondii type I ROP18 in human cells.Parasitology. 2020 Nov;147(13):1433-1442. doi: 10.1017/S0031182020001249. Epub 2020 Jul 30. Parasitology. 2020. PMID: 32729455 Free PMC article.
-
Functional Analysis of the Rhoptry Kinome during Chronic Toxoplasma gondii Infection.mBio. 2016 Jun 14;7(3):e00842-16. doi: 10.1128/mBio.00842-16. mBio. 2016. PMID: 27302762 Free PMC article.
-
The Expressed MicroRNA-mRNA Interactions of Toxoplasma gondii.Front Microbiol. 2018 Jan 4;8:2630. doi: 10.3389/fmicb.2017.02630. eCollection 2017. Front Microbiol. 2018. PMID: 29354114 Free PMC article.
-
T. gondii rhoptry protein ROP18 induces apoptosis of neural cells via endoplasmic reticulum stress pathway.Parasit Vectors. 2015 Oct 21;8:554. doi: 10.1186/s13071-015-1103-z. Parasit Vectors. 2015. PMID: 26489755 Free PMC article.
-
Rhoptry antigens as Toxoplasma gondii vaccine target.Clin Exp Vaccine Res. 2019 Jan;8(1):4-26. doi: 10.7774/cevr.2019.8.1.4. Epub 2019 Jan 31. Clin Exp Vaccine Res. 2019. PMID: 30775347 Free PMC article. Review.
References
-
- Bastin P, Bagherzadeh Z, Matthews KR, Gull K. A novel epitope tag system to study protein targeting and organelle biogenesis in Trypanosoma brucei. Molec. Biochem. Parasitol. 1996;77:235–239. - PubMed
-
- Betts HJ, Wolf K, Fields KA. Effector protein modulation of host cells: examples in the Chlamydia spp. arsenal. Curr Opin Microbiol. 2009;12:81–87. - PubMed
-
- Boothroyd JC, Dubremetz JF. Kiss and spit: the dual roles of Toxoplasma rhoptries. Nat. Rev. Microbiol. 2008;6:79–88. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

