Introducing D-amino acid or simple glycoside into small peptides to enable supramolecular hydrogelators to resist proteolysis
- PMID: 22906360
- PMCID: PMC3472800
- DOI: 10.1021/la302583a
Introducing D-amino acid or simple glycoside into small peptides to enable supramolecular hydrogelators to resist proteolysis
Abstract
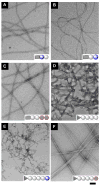
Here we report the examination of two convenient strategies, the use of a d-amino acid residue or a glycoside segment, for increasing the proteolytic resistance of supramolecular hydrogelators based on small peptides. Our results show that the introduction of d-amino acid or glycoside to the peptides significantly increases the resistance of the hydrogelators against proteinase K, a powerful endopeptidase. The insertion of d-amino acid in the peptide backbone, however, results relatively low storage moduli of the hydrogels, likely due to the disruption of the superstructures of the molecular assembly. In contrast, the introduction of a glycoside to the C-terminal of peptide enhances the biostability of the hydrogelators without the significant decrease of the storage moduli of the hydrogels. This work suggests that the inclusion of a simple glycogen in hydrogelators is a useful approach to increase their biostability, and the gained understanding from the work may ultimately lead to development of hydrogels of functional peptides for biomedical applications that require long-term biostability.
Figures




Similar articles
-
Multifunctional, biocompatible supramolecular hydrogelators consist only of nucleobase, amino acid, and glycoside.J Am Chem Soc. 2011 Nov 2;133(43):17513-8. doi: 10.1021/ja208456k. Epub 2011 Oct 7. J Am Chem Soc. 2011. PMID: 21928792 Free PMC article.
-
D-amino acids modulate the cellular response of enzymatic-instructed supramolecular nanofibers of small peptides.Biomacromolecules. 2014 Oct 13;15(10):3559-68. doi: 10.1021/bm5010355. Epub 2014 Sep 17. Biomacromolecules. 2014. PMID: 25230147 Free PMC article.
-
Supramolecular Hydrogelators and Hydrogels: From Soft Matter to Molecular Biomaterials.Chem Rev. 2015 Dec 23;115(24):13165-307. doi: 10.1021/acs.chemrev.5b00299. Epub 2015 Dec 8. Chem Rev. 2015. PMID: 26646318 Free PMC article. Review.
-
Dephosphorylation of D-peptide derivatives to form biofunctional, supramolecular nanofibers/hydrogels and their potential applications for intracellular imaging and intratumoral chemotherapy.J Am Chem Soc. 2013 Jul 3;135(26):9907-14. doi: 10.1021/ja404215g. Epub 2013 Jun 21. J Am Chem Soc. 2013. PMID: 23742714 Free PMC article.
-
Supramolecular hydrogels made of basic biological building blocks.Chem Asian J. 2014 Jun;9(6):1446-72. doi: 10.1002/asia.201301693. Epub 2014 Mar 12. Chem Asian J. 2014. PMID: 24623474 Free PMC article. Review.
Cited by
-
Mapping enzyme activity in living systems by real-time mid-infrared photothermal imaging of nitrile chameleons.Nat Methods. 2024 Feb;21(2):342-352. doi: 10.1038/s41592-023-02137-x. Epub 2024 Jan 8. Nat Methods. 2024. PMID: 38191931 Free PMC article.
-
The first CD73-instructed supramolecular hydrogel.J Colloid Interface Sci. 2015 Jun 1;447:269-72. doi: 10.1016/j.jcis.2014.11.050. Epub 2014 Nov 25. J Colloid Interface Sci. 2015. PMID: 25524006 Free PMC article.
-
Supramolecular biofunctional materials.Biomaterials. 2017 Jun;129:1-27. doi: 10.1016/j.biomaterials.2017.03.014. Epub 2017 Mar 12. Biomaterials. 2017. PMID: 28319779 Free PMC article. Review.
-
Harnessing the potential of hydrogels for advanced therapeutic applications: current achievements and future directions.Signal Transduct Target Ther. 2024 Jul 1;9(1):166. doi: 10.1038/s41392-024-01852-x. Signal Transduct Target Ther. 2024. PMID: 38945949 Free PMC article. Review.
-
Designing Coiled Coils for Heterochiral Complexation to Enhance Binding and Enzymatic Stability.Biomacromolecules. 2024 Aug 12;25(8):5273-5280. doi: 10.1021/acs.biomac.4c00661. Epub 2024 Jul 9. Biomacromolecules. 2024. PMID: 38980285 Free PMC article.
References
-
- Terech P, Weiss RG. Chem Rev. 1997;97:3133–3159. - PubMed
- Estroff LA, Hamilton AD. Chem Rev. 2004;104:1201–1217. - PubMed
- Hirst AR, Coates IA, Boucheteau TR, Miravet JF, Escuder B, Castelletto V, Hamley IW, Smith DK. J Am Chem Soc. 2008;130:9113–9121. - PubMed
- Sahoo P, Adarsh NN, Chacko GE, Raghavan SR, Puranik VG, Dastidar P. Langmuir. 2009;25:8742–8750. - PubMed
- Suzuki M, Hanabusa K. Chem Soc Rev. 2009;38:967–975. - PubMed
- Chen L, Morris K, Laybourn A, Elias D, Hicks MR, Rodger A, Serpell L, Adams DJ. Langmuir. 2010;26:5232–5242. - PubMed
- Yan C, Pochan DJ. Chem Soc Rev. 2010;39:3528–3540. - PMC - PubMed
- Dawn A, Shiraki T, Haraguchi S, Tamaru S, Shinkai S. Chem Asian J. 2011;6:266–282. - PubMed
- Steed JW. Chem Commun. 2011;47:1379–1383. - PubMed
- Das D, Kar T, Das PK. Soft Matter. 2012;8:2348–2365.
- Yan C, Mackay ME, Czymmek K, Nagarkar RP, Schneider JP, Pochan DJ. Langmuir. 2012;28:6076–6087. - PMC - PubMed
-
- Zhang SG. Nat Biotechnol. 2003;21:1171–1178. - PubMed
-
- Rajagopal K, Schneider JP. Curr Opin Struct Biol. 2004;14:480–486. - PubMed
- Ulijn RV, Smith AM. Chem Soc Rev. 2008;37:664–675. - PubMed
- Yang Z, Liang G, Xu B. Acc Chem Res. 2008;41:315–326. - PubMed
- Cui H, Webber MJ, Stupp SI. Biopolymers. 2010;94:1–18. - PMC - PubMed
- Matson JB, Stupp SI. Chem Commun. 2012;48:26–33. - PMC - PubMed
-
- Yang Z, Xu B. J Mater Chem. 2007;17:2385–2393.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

