Collagen XIV is important for growth and structural integrity of the myocardium
- PMID: 22906538
- PMCID: PMC3472103
- DOI: 10.1016/j.yjmcc.2012.08.002
Collagen XIV is important for growth and structural integrity of the myocardium
Abstract
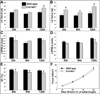
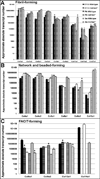
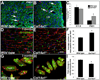
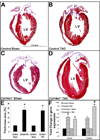
Collagen XIV is a fibril-associated collagen with an interrupted triple helix (FACIT). Previous studies have shown that this collagen type regulates early stages of fibrillogenesis in connective tissues of high mechanical demand. Mice null for Collagen XIV are viable, however formation of the interstitial collagen network is defective in tendons and skin leading to reduced biomechanical function. The assembly of a tightly regulated collagen network is also required in the heart, not only for structural support but also for controlling cellular processes. Collagen XIV is highly expressed in the embryonic heart, notably within the cardiac interstitium of the developing myocardium, however its role has not been elucidated. To test this, we examined cardiac phenotypes in embryonic and adult mice devoid of Collagen XIV. From as early as E11.5, Col14a1(-/-) mice exhibit significant perturbations in mRNA levels of many other collagen types and remodeling enzymes (MMPs, TIMPs) within the ventricular myocardium. By post natal stages, collagen fibril organization is in disarray and the adult heart displays defects in ventricular morphogenesis. In addition to the extracellular matrix, Col14a1(-/-) mice exhibit increased cardiomyocyte proliferation at post natal, but not E11.5 stages, leading to increased cell number, yet cell size is decreased by 3 months of age. In contrast to myocytes, the number of cardiac fibroblasts is reduced after birth associated with increased apoptosis. As a result of these molecular and cellular changes during embryonic development and post natal maturation, cardiac function is diminished in Col14a1(-/-) mice from 3 months of age; associated with dilation in the absence of hypertrophy, and reduced ejection fraction. Further, Col14a1 deficiency leads to a greater increase in left ventricular wall thickening in response to pathological pressure overload compared to wild type animals. Collectively, these studies identify a new role for type XIV collagen in the formation of the cardiac interstitium during embryonic development, and highlight the importance of the collagen network for myocardial cell survival, and function of the working myocardium after birth.
Copyright © 2012 Elsevier Ltd. All rights reserved.
Conflict of interest statement
Figures




References
-
- Eghbali M, Weber KT. Collagen and the myocardium: fibrillar structure, biosynthesis and degradation in relation to hypertrophy and its regression. Molecular and cellular biochemistry. 1990;96:1–14. - PubMed
-
- Carver W, Terracio L, Borg TK. Expression and accumulation of interstitial collagen in the neonatal rat heart. Anat Rec. 1993;236:511–520. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

