Seasonal maintenance of influenza vaccine-induced antibody response in kidney transplant recipients
- PMID: 22906930
- PMCID: PMC3482977
- DOI: 10.1159/000341653
Seasonal maintenance of influenza vaccine-induced antibody response in kidney transplant recipients
Abstract
Background/aims: Although annual influenza vaccination is recommended for kidney transplant recipients, efficacy as reflected by serum antibody titers has not been well studied beyond 1 month in kidney transplant recipients.
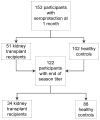
Methods: We performed a single-center prospective cohort study of 51 kidney transplant recipients and 102 healthy controls receiving the 2006-2007 influenza vaccine. Anti-hemagglutinin antibody titers to A/H1N1, A/H3N2, and B were measured before and 1 month after vaccination, and again at the end of influenza season. The primary outcome was the proportion of participants maintaining seroprotection (antibody titer ≥1:32) for the duration of the influenza season after influenza vaccination.
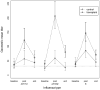
Results: Median follow-up time was 175 and 155 days in the transplant and control groups, respectively. For types A/H1N1 and B, a similar high proportion of the transplant and control groups (88.5 and 81.6% vs. 83.7 and 74.2% for A/H1N1 and B, respectively) maintained seroprotection. For type A/H3N2, significantly less of the transplant group (66.7%) versus the control group (90%) maintained a protective influenza vaccine response (odds ratio 0.21, 95% confidence interval 0.07-0.64). This difference disappeared in adjusted analyses. Actual geometric mean titers decreased significantly within both groups (p < 0.001) but this did not differ between groups.
Conclusions: Once they have developed protective vaccine-induced antibody responses to influenza vaccine, kidney transplant recipients are able to maintain adequate protective levels of antibody compared with healthy controls.
Copyright © 2012 S. Karger AG, Basel.
Conflict of interest statement
Financial Conflicts of Interest: None of the authors report any conflicts of interest.
Kelly A. Birdwell, MD, MSCI: None
Mine R. Ikizler, MS: None
Edith C. Sannella, MT: None
Li Wang, MS: None
Daniel W. Byrne, MS: None
Peter F. Wright, MD: None
T. Alp Ikizler, MD: None
Figures
References
-
- Kotton CN, Fishman JA. Viral infection in the renal transplant recipient. J Am Soc Nephrol. 2005;16:1758–1774. - PubMed
-
- Danzinger-Isakov L, Kumar D. Guidelines for vaccination of solid organ transplant candidates and recipients. Am J Transplant. 2009;9 (Suppl 4):S258–262. - PubMed
-
- Fiore AE, Uyeki TM, Broder K, Finelli L, Euler GL, Singleton JA, Iskander JK, Wortley PM, Shay DK, Bresee JS, Cox NJ. Prevention and control of influenza with vaccines: recommendations of the Advisory Committee on Immunization Practices (ACIP), 2010. MMWR Recomm Rep. 2010;59:1–62. - PubMed
-
- Vilchez RA, Fung J, Kusne S. The pathogenesis and management of influenza virus infection in organ transplant recipients. Transpl Infect Dis. 2002;4:177–182. - PubMed
-
- Edwards KM, Dupont WD, Westrich MK, Plummer WD, Jr, Palmer PS, Wright PF. A randomized controlled trial of cold-adapted and inactivated vaccines for the prevention of influenza A disease. J Infect Dis. 1994;169:68–76. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

