Mechanistically linking age-related diseases and dietary carbohydrate via autophagy and the ubiquitin proteolytic systems
- PMID: 22906982
- PMCID: PMC3442892
- DOI: 10.4161/auto.21150
Mechanistically linking age-related diseases and dietary carbohydrate via autophagy and the ubiquitin proteolytic systems
Abstract
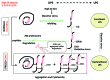
Epidemiological data indicate that consuming diets that deliver sugar to the blood rapidly (called high glycemic index, GI) is associated with enhanced risk for age-related diseases such as cardiovascular disease, type 2 diabetes, cataract and age-related macular degeneration (AMD). These debilities are associated with accumulation of toxic protein aggregates as observed in other protein precipitation or amyloid diseases including Alzheimer, Parkinson and Huntington diseases and encephalopathies. Barriers to recommending lower-GI diets to promote health include the absence of established intracellular biochemical mechanisms that link high-GI diets to compromised homeostasis. The data herein corroborate the epidemiological findings and provide platforms to elucidate additional mechanistic aspects of salutary effects of consuming diets of different GIs. They are also useful for testing drugs, including autophagy enhancers, glycemia regulators, or nutraceuticals, which can be exploited to extend health.
Figures
Comment on
- Uchiki T, Weikel KA, Jiao W, Shang F, Caceres A, Pawlak D, et al. Glycation-altered proteolysis as a pathobiologic mechanism that links dietary glycemic index, aging, and age-related disease (in nondiabetics) Aging Cell. 2012;11:1–13. doi: 10.1111/j.1474-9726.2011.00752.x. doi: 10.1111/j.1474-9726.2011.00752.x
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
