Monoamine oxidase A expression is suppressed in human cholangiocarcinoma via coordinated epigenetic and IL-6-driven events
- PMID: 22906985
- PMCID: PMC3959781
- DOI: 10.1038/labinvest.2012.110
Monoamine oxidase A expression is suppressed in human cholangiocarcinoma via coordinated epigenetic and IL-6-driven events
Abstract
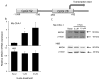
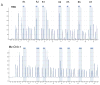
The secretion of dopamine and serotonin is increased in cholangiocarcinoma, which has growth-promoting effects. Monoamine oxidase A (MAOA), the degradation enzyme of serotonin and dopamine, is suppressed in cholangiocarcinoma via an unknown mechanism. The aims of this study were to (i) correlate MAOA immunoreactivity with pathophysiological parameters of cholangiocarcinoma, (ii) determine the mechanism by which MAOA expression is suppressed and (iii) evaluate the consequences of restored MAOA expression in cholangiocarcinoma. MAOA expression was assessed in cholangiocarcinoma and nonmalignant controls. The control of MAOA expression by promoter hypermethylation was evaluated and the contribution of interleukin-6 (IL-6) signaling to the suppression of MAOA expression was determined. The effects of MAOA overexpression on cholangiocarcinoma growth and invasion were also assessed. MAOA expression is correlated with differentiation, invasion and survival in cholangiocarcinoma. The MAOA promoter was hypermethylated immediately upstream of the start codon in cholangiocarcinoma samples and cell lines but not in nonmalignant counterparts. IL-6 signaling also decreased MAOA expression via a mechanism independent of hypermethylation, involving the regulation of the balance between SP-1 transcriptional activity and its inhibitor, R1 repressor. Inhibition of both IL-6 signaling and DNA methylation restored MAOA levels to those observed in cholangiocytes. Forced MAOA overexpression inhibited cholangiocarcinoma growth and invasion. MAOA expression is suppressed by the coordinated control of promoter hypermethylation and IL-6 signaling. MAOA may be a useful prognostic marker in the management of cholangiocarcinoma, and therapies designed to increase MAOA expression might prove beneficial in the treatment of cholangiocarcinoma.
Figures
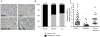
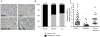



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

