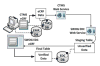
The automatic clinical trial: leveraging the electronic medical record in multisite cancer clinical trials
- PMID: 22907283
- PMCID: PMC3490046
- DOI: 10.1007/s11912-012-0262-8
The automatic clinical trial: leveraging the electronic medical record in multisite cancer clinical trials
Abstract
Submission of data into clinical trial electronic data capture (EDC) systems currently requires redundant entry of data that already exist in the electronic medical record (EMR). Being able to automatically transfer data from the EMR to the EDC system would save many hours of arduous effort, especially for multisite data-intensive oncology trials. Standardization of the way in which data are stored in and retrieved from the EMR and techniques for mining data from the unstructured narrative will provide opportunities for transferring data from the EMR to the EDC system. As different EMRs proliferate, other technology in the form of data mining or middle-tier applications is certain to provide assistance in this effort.
Conflict of interest statement
No potential conflicts of interest relevant to this article were reported.
Figures
References
-
- Rogers J. EDC & EHR integration. Appl Clin Trials. 2010;19:52–56.
-
- Mitchel JT, Yong Joong K, Joonhyuk C, et al. The final eFrontier. Appl Clinl Trials. 2010;19:44–50.
-
- McIlwain J. A to Z trial integration. Appl Clin Trials. 2007;16:48–56.
-
- D’Avolio LW. Electronic medical records at a crossroads. impetus for change or missed opportunity? JAMA. 2009;302:1109–1111. - PubMed
-
- Kanas G, Morimoto L, Mowat F, et al. Use of electronic medical records in oncology outcomes research. Clinicoecon Outcomes Res. 2010;2:1–14. Use of the electronic medical record (EMR) in oncology outcomes research can provide comprehensive and accurate information on all areas of patient care. An oncology-specific EMR system must include structural, clinical, and research-related issues. - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials