A new generation of human artificial chromosomes for functional genomics and gene therapy
- PMID: 22907415
- PMCID: PMC3522797
- DOI: 10.1007/s00018-012-1113-3
A new generation of human artificial chromosomes for functional genomics and gene therapy
Abstract
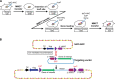
Since their description in the late 1990s, human artificial chromosomes (HACs) carrying a functional kinetochore were considered as a promising system for gene delivery and expression with a potential to overcome many problems caused by the use of viral-based gene transfer systems. Indeed, HACs avoid the limited cloning capacity, lack of copy number control and insertional mutagenesis due to integration into host chromosomes that plague viral vectors. Nevertheless, until recently, HACs have not been widely recognized because of uncertainties of their structure and the absence of a unique gene acceptor site. The situation changed a few years ago after engineering of HACs with a single loxP gene adopter site and a defined structure. In this review, we summarize recent progress made in HAC technology and concentrate on details of two of the most advanced HACs, 21HAC generated by truncation of human chromosome 21 and alphoid(tetO)-HAC generated de novo using a synthetic tetO-alphoid DNA array. Multiple potential applications of the HAC vectors are discussed, specifically the unique features of two of the most advanced HAC cloning systems.
Figures





References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

