Rational development of a serum-free medium and fed-batch process for a GS-CHO cell line expressing recombinant antibody
- PMID: 22907508
- PMCID: PMC3597181
- DOI: 10.1007/s10616-012-9488-4
Rational development of a serum-free medium and fed-batch process for a GS-CHO cell line expressing recombinant antibody
Abstract
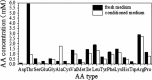
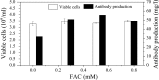
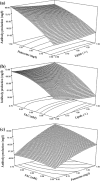
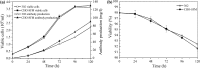
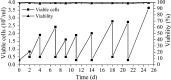
A serum-free medium (CHO-SFM) together with a fed-batch process was developed for the cultivation of a recombinant GS-CHO cell line producing TNFR-Fc. According to the metabolic characteristics of GS-CHO cell, a basal medium was prepared by supplementing DMEM:F12:RPMI1640 (2:1:1) with amino acids, insulin, transferrin, Pluronic F68 and some other ingredients. Statistical optimization approaches based on Plackett-Burman and central composite designs were then adopted to identify additional positive determinants and determine their optimal concentrations, which resulted in the final CHO-SFM medium formulations. The maximum antibody titer reached was 90.95 mg/l in the developed CHO-SFM, which was a 18 % and 10 fold higher than that observed in the commercial EX-CELL™ 302 medium (76.95 mg/l) and basal medium (8.28 mg/l), respectively. Subsequently, a reliable, reproducible and robust fed-batch strategy was designed according to the offline measurement of glucose, giving a final antibody yield of 378 mg/l, which was a threefold improvement over that in conventional batch culture (122 mg/l) using CHO-SFM. In conclusion, the use of design of experiment (DoE) method facilitated the development of CHO-SFM medium and fed-batch process for the production of recombinant antibody using GS-CHO cells.
Figures

References
-
- Abdeen SH, Abdeen AM, EI-Enshasy HA, Shereef AAE (2011) HeLa-S3 cell growth conditions in serum-free medium and adaptability for proliferation in suspension culture. J Biol Sci 11:124–134
-
- Bathon JM, Martin RW, Fleischmann RM, Tesser JR, Schiff MH, Keystone EC, Genovese MC, Wasko MC, Moreland LW, Weaver AL, Markenson J, Finck BK. A comparison of etanercept and methotrexate in patients with early rheumatoid arthritis. N Engl J Med. 2000;343:1586–1593. doi: 10.1056/NEJM200011303432201. - DOI - PubMed
-
- Box GEP, Hunter WG, Hunter JS. Fractional factorial design at two levels. In: Statistics for experimenters. New York: Wiley; 1978. pp. 374–418.
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

