Rethinking WHO guidance: review of evidence for misoprostol use in the prevention of postpartum haemorrhage
- PMID: 22907551
- PMCID: PMC3423133
- DOI: 10.1258/jrsm.2012.120044
Rethinking WHO guidance: review of evidence for misoprostol use in the prevention of postpartum haemorrhage
Erratum in
- J R Soc Med. 2012 Nov;105(11):456
Abstract
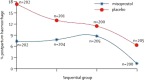
This article describes and critically appraises clinical trials assessing misoprostol effectiveness in preventing primary postpartum haemorrhage (PPH) in home and community settings in low- and middle-income countries. Of 172 identified studies of misoprostol use in labour only six fulfilled the inclusion criteria. All trials used 600 μg misoprostol in the intervention arm; three assessed misoprostol alongside components of active management of the third-stage labour (AMTSL), two used expectant management of labour and one allowed birth attendants to choose management practice. The three AMTSL studies showed no significant differences in PPH incidence or referral to higher centres and only one study showed significant decrease in severe PPH using misoprostol. One expectant management study and the choice of management by birth attendants study found significant decreases in PPH incidence with misoprostol. All studies showed significantly increased risk of shivering with misoprostol. Studies were biased by use of alternative uterotonics in the control arm, confounding management practices, and subjective assessment and, with one exception, exclusion of high-risk women. PPH incidence fell in both the control and intervention groups in both the landmark papers that informed the World Health Organization (WHO) decision to admit misoprostol to the Essential Medicines List. This suggests factors other than misoprostol use are crucial. Current evidence does not support misoprostol use in home and community settings in low- and middle-income countries for PPH prevention. WHO should rethink its recent decision to include misoprostol on the Essential Medicines List.
Figures
Comment in
-
No excuse for ignoring methodologically sound studies.J R Soc Med. 2012 Nov;105(11):455. doi: 10.1258/JRSM.2012.12K082. J R Soc Med. 2012. PMID: 23257965 Free PMC article. No abstract available.
Similar articles
-
Should we be using misoprostol to prevent postpartum haemorrhage?Pract Midwife. 2013 May;16(5):9. Pract Midwife. 2013. PMID: 23789247 No abstract available.
-
Misoprostol for the prevention and treatment of postpartum haemorrhage.Best Pract Res Clin Obstet Gynaecol. 2008 Dec;22(6):1025-41. doi: 10.1016/j.bpobgyn.2008.08.005. Epub 2008 Sep 10. Best Pract Res Clin Obstet Gynaecol. 2008. PMID: 18786863 Review.
-
Misoprostol for postpartum hemorrhage prevention at home birth: an integrative review of global implementation experience to date.BMC Pregnancy Childbirth. 2013 Feb 20;13:44. doi: 10.1186/1471-2393-13-44. BMC Pregnancy Childbirth. 2013. PMID: 23421792 Free PMC article. Review.
-
Effectiveness and safety of misoprostol distributed to antenatal women to prevent postpartum haemorrhage after child-births: a stepped-wedge cluster-randomized trial.BMC Pregnancy Childbirth. 2015 Nov 26;15:315. doi: 10.1186/s12884-015-0750-6. BMC Pregnancy Childbirth. 2015. PMID: 26610333 Free PMC article. Clinical Trial.
-
Misoprostol and active management of the third stage of labor.Int J Gynaecol Obstet. 2006 Aug;94(2):149-55. doi: 10.1016/j.ijgo.2006.05.027. Epub 2006 Jul 7. Int J Gynaecol Obstet. 2006. PMID: 16828767 Clinical Trial.
Cited by
-
Potential Cost-Effectiveness of Prenatal Distribution of Misoprostol for Prevention of Postpartum Hemorrhage in Uganda.PLoS One. 2015 Nov 11;10(11):e0142550. doi: 10.1371/journal.pone.0142550. eCollection 2015. PLoS One. 2015. PMID: 26560140 Free PMC article.
-
Misoprostol for Prevention of Postpartum Hemorrhage at Home Birth in Afghanistan: Program Expansion Experience.J Midwifery Womens Health. 2016 Mar-Apr;61(2):196-202. doi: 10.1111/jmwh.12413. Epub 2016 Feb 5. J Midwifery Womens Health. 2016. PMID: 26849472 Free PMC article.
-
Improving WHO's understanding of WHO guideline uptake and use in Member States: a scoping review.Health Res Policy Syst. 2022 Sep 7;20(1):98. doi: 10.1186/s12961-022-00899-y. Health Res Policy Syst. 2022. PMID: 36071468 Free PMC article.
-
Misoprostol to reduce intraoperative and postoperative hemorrhage during cesarean delivery: a systematic review and metaanalysis.Am J Obstet Gynecol. 2013 Jul;209(1):40.e1-40.e17. doi: 10.1016/j.ajog.2013.03.015. Epub 2013 Mar 15. Am J Obstet Gynecol. 2013. PMID: 23507545 Free PMC article.
-
Barriers or gaps in implementation of misoprostol use for post-abortion care and post-partum hemorrhage prevention in developing countries: a systematic review.Reprod Health. 2017 Oct 27;14(1):139. doi: 10.1186/s12978-017-0383-5. Reprod Health. 2017. PMID: 29078777 Free PMC article.
References
-
- Hogan M, Foreman K, Naghavi M, et al. Maternal mortality for 181 countries, 1980–2008: a systematic analysis of progress towards Millennium Development Goal 5. Lancet 2010;375:1609–23 - PubMed
-
- Hill K, Thomas K, Abou Zahr C, et al. Estimates of maternal mortality worldwide between 1990 and 2005: an assessment of available data. Lancet 2007;370:1311–9 - PubMed
-
- WHO The World Health Report 2005. Make every mother and child count 2005 See http://www.who.int/whr/2005/whr2005_en.pdf (last accessed 3 July 2012)
-
- Brabin B, Hakimi M, Pelletier D An analysis of anemia and pregnancy-related maternal mortality. J Nutr 2001;131:604S–14S - PubMed
-
- WHO Worldwide Prevalence of Anaemia 1993–2005: WHO Global Database on Anaemia. Geneva: World Health Organisation, 2008
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources