The 5-phosphatase OCRL mediates retrograde transport of the mannose 6-phosphate receptor by regulating a Rac1-cofilin signalling module
- PMID: 22907655
- PMCID: PMC3490508
- DOI: 10.1093/hmg/dds343
The 5-phosphatase OCRL mediates retrograde transport of the mannose 6-phosphate receptor by regulating a Rac1-cofilin signalling module
Abstract
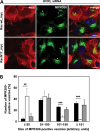
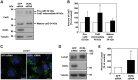
Mutations in the OCRL gene encoding the phosphatidylinositol 4,5-bisphosphate (PI(4,5)P(2)) 5-phosphatase OCRL cause Lowe syndrome (LS), which is characterized by intellectual disability, cataracts and selective proximal tubulopathy. OCRL localizes membrane-bound compartments and is implicated in intracellular transport. Comprehensive analysis of clathrin-mediated endocytosis in fibroblasts of patients with LS did not reveal any difference in trafficking of epidermal growth factor, low density lipoprotein or transferrin, compared with normal fibroblasts. However, LS fibroblasts displayed reduced mannose 6-phosphate receptor (MPR)-mediated re-uptake of the lysosomal enzyme arylsulfatase B. In addition, endosome-to-trans Golgi network (TGN) transport of MPRs was decreased significantly, leading to higher levels of cell surface MPRs and their enrichment in enlarged, retromer-positive endosomes in OCRL-depleted HeLa cells. In line with the higher steady-state concentration of MPRs in the endosomal compartment in equilibrium with the cell surface, anterograde transport of the lysosomal enzyme, cathepsin D was impaired. Wild-type OCRL counteracted accumulation of MPR in endosomes in an activity-dependent manner, suggesting that PI(4,5)P(2) modulates the activity state of proteins regulated by this phosphoinositide. Indeed, we detected an increased amount of the inactive, phosphorylated form of cofilin and lower levels of the active form of PAK3 upon OCRL depletion. Levels of active Rac1 and RhoA were reduced or enhanced, respectively. Overexpression of Rac1 rescued both enhanced levels of phosphorylated cofilin and MPR accumulation in enlarged endosomes. Our data suggest that PI(4,5)P(2) dephosphorylation through OCRL regulates a Rac1-cofilin signalling cascade implicated in MPR trafficking from endosomes to the TGN.
Figures










Similar articles
-
Rab35 GTPase Triggers Switch-like Recruitment of the Lowe Syndrome Lipid Phosphatase OCRL on Newborn Endosomes.Curr Biol. 2016 Jan 11;26(1):120-8. doi: 10.1016/j.cub.2015.11.040. Epub 2015 Dec 24. Curr Biol. 2016. PMID: 26725203
-
Interaction of Rab31 and OCRL-1 in oligodendrocytes: its role in transport of mannose 6-phosphate receptors.J Neurosci Res. 2010 Feb 15;88(3):589-604. doi: 10.1002/jnr.22236. J Neurosci Res. 2010. PMID: 19795375 Free PMC article.
-
Lowe syndrome protein OCRL1 interacts with clathrin and regulates protein trafficking between endosomes and the trans-Golgi network.Mol Biol Cell. 2005 Aug;16(8):3467-79. doi: 10.1091/mbc.e05-02-0120. Epub 2005 May 25. Mol Biol Cell. 2005. PMID: 15917292 Free PMC article.
-
The role of the Lowe syndrome protein OCRL in the endocytic pathway.Biol Chem. 2015 Dec;396(12):1293-300. doi: 10.1515/hsz-2015-0180. Biol Chem. 2015. PMID: 26351914 Review.
-
Visualization of TGN-endosome trafficking in mammalian and Drosophila cells.Methods Enzymol. 2012;504:255-71. doi: 10.1016/B978-0-12-391857-4.00013-6. Methods Enzymol. 2012. PMID: 22264539 Review.
Cited by
-
Insulin-Like Growth Factor 2 As a Possible Neuroprotective Agent and Memory Enhancer-Its Comparative Expression, Processing and Signaling in Mammalian CNS.Int J Mol Sci. 2021 Feb 12;22(4):1849. doi: 10.3390/ijms22041849. Int J Mol Sci. 2021. PMID: 33673334 Free PMC article. Review.
-
MicroRNA 199a-5p Attenuates Retrograde Transport and Protects against Toxin-Induced Inhibition of Protein Biosynthesis.Mol Cell Biol. 2018 May 15;38(11):e00548-17. doi: 10.1128/MCB.00548-17. Print 2018 Jun 1. Mol Cell Biol. 2018. PMID: 29555727 Free PMC article.
-
Rho GTPases, phosphoinositides, and actin: a tripartite framework for efficient vesicular trafficking.Small GTPases. 2014;5:e29469. doi: 10.4161/sgtp.29469. Epub 2014 Jun 10. Small GTPases. 2014. PMID: 24914539 Free PMC article. Review.
-
A missense mutant of ocrl1 promotes apoptosis of tubular epithelial cells and disrupts endocytosis and the cell cycle of podocytes in Dent-2 Disease.Cell Commun Signal. 2023 Dec 5;21(1):256. doi: 10.1186/s12964-023-01272-4. Cell Commun Signal. 2023. PMID: 38049819 Free PMC article.
-
Protein sorting from endosomes to the TGN.Front Cell Dev Biol. 2023 Feb 21;11:1140605. doi: 10.3389/fcell.2023.1140605. eCollection 2023. Front Cell Dev Biol. 2023. PMID: 36895788 Free PMC article. Review.
References
-
- Lowe C.U., Terrey M., Mac L.E. Organic-aciduria, decreased renal ammonia production, hydrophthalmos, and mental retardation; a clinical entity. AMA Am. J. Dis. Child. 1952;83:164–184. - PubMed
-
- Loi M. Lowe syndrome. Orphanet J. Rare Dis. 2006;1:16. doi:10.1186/1750-1172-1-16. - DOI - PMC - PubMed
-
- Bockenhauer D., Bokenkamp A., van't Hoff W., Levtchenko E., Kist-van Holthe J.E., Tasic V., Ludwig M. Renal phenotype in Lowe Syndrome: a selective proximal tubular dysfunction. Clin. J. Am. Soc. Nephrol. 2008;3:1430–1436. doi:10.2215/CJN.00520108. - DOI - PMC - PubMed
-
- Attree O., Olivos I.M., Okabe I., Bailey L.C., Nelson D.L., Lewis R.A., McInnes R.R., Nussbaum R.L. The Lowe's oculocerebrorenal syndrome gene encodes a protein highly homologous to inositol polyphosphate-5-phosphatase. Nature. 1992;358:239–242. doi:10.1038/358239a0. - DOI - PubMed
-
- Nussbaum R.L., Orrison B.M., Janne P.A., Charnas L., Chinault A.C. Physical mapping and genomic structure of the Lowe syndrome gene OCRL1. Hum. Genet. 1997;99:145–150. doi:10.1007/s004390050329. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

