The prolyl isomerase Pin1 targets stem-loop binding protein (SLBP) to dissociate the SLBP-histone mRNA complex linking histone mRNA decay with SLBP ubiquitination
- PMID: 22907757
- PMCID: PMC3486140
- DOI: 10.1128/MCB.00382-12
The prolyl isomerase Pin1 targets stem-loop binding protein (SLBP) to dissociate the SLBP-histone mRNA complex linking histone mRNA decay with SLBP ubiquitination
Abstract
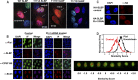
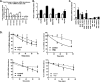
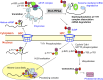
Histone mRNAs are rapidly degraded at the end of S phase, and a 26-nucleotide stem-loop in the 3' untranslated region is a key determinant of histone mRNA stability. This sequence is the binding site for stem-loop binding protein (SLBP), which helps to recruit components of the RNA degradation machinery to the histone mRNA 3' end. SLBP is the only protein whose expression is cell cycle regulated during S phase and whose degradation is temporally correlated with histone mRNA degradation. Here we report that chemical inhibition of the prolyl isomerase Pin1 or downregulation of Pin1 by small interfering RNA (siRNA) increases the mRNA stability of all five core histone mRNAs and the stability of SLBP. Pin1 regulates SLBP polyubiquitination via the Ser20/Ser23 phosphodegron in the N terminus. siRNA knockdown of Pin1 results in accumulation of SLBP in the nucleus. We show that Pin1 can act along with protein phosphatase 2A (PP2A) in vitro to dephosphorylate a phosphothreonine in a conserved TPNK sequence in the SLBP RNA binding domain, thereby dissociating SLBP from the histone mRNA hairpin. Our data suggest that Pin1 and PP2A act to coordinate the degradation of SLBP by the ubiquitin proteasome system and the exosome-mediated degradation of the histone mRNA by regulating complex dissociation.
Figures

References
-
- Albert A, Lavoie S, Vincent M. 1999. A hyperphosphorylated form of RNA polymerase II is the major interphase antigen of the phosphoprotein antibody MPM-2 and interacts with the peptidyl-prolyl isomerase Pin1. J. Cell Sci. 112(Part 15):2493–2500 - PubMed
-
- Dimitrova DS, Berezney R. 2002. The spatio-temporal organization of DNA replication sites is identical in primary, immortalized and transformed mammalian cells. J. Cell Sci. 115:4037–4051 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous
