Matrix tablets: the effect of hydroxypropyl methylcellulose/anhydrous dibasic calcium phosphate ratio on the release rate of a water-soluble drug through the gastrointestinal tract I. In vitro tests
- PMID: 22907778
- PMCID: PMC3513448
- DOI: 10.1208/s12249-012-9829-9
Matrix tablets: the effect of hydroxypropyl methylcellulose/anhydrous dibasic calcium phosphate ratio on the release rate of a water-soluble drug through the gastrointestinal tract I. In vitro tests
Abstract
Different hydroxypropyl methylcellulose (HPMC)/anhydrous dibasic calcium phosphate (ADCP) matrix tablets have been developed aiming to evaluate the influence of both components ratio in the control release of a water-soluble drug (theophylline). In order to characterise the matrix tablets, swelling, buoyancy and dissolution studies have been carried out in different aqueous media (demineralised water, progressive pH medium, simulated gastric fluid, simulated intestinal fluid and simulated colonic fluid). The HPMC/ADCP ratio has turned out to be the determinant in the matrix behaviour: the HPMC characteristic swelling behaviour was modulated, in some cases, by the ADCP characteristic acidic dissolution. When the HPMC/ADCP ratio was ≥0.69, buoyancy, continuous swelling and low theophylline dissolution rate from the matrices (H1, H2 and H3) were observed in all dissolution media. Consequently, these formulations could be adequate as gastro-retentive drug delivery systems. Additionally, HPMC/ADCP ratio ≤0.11 (H5 and H6) induces a pH-dependent drug release which could be applied to design control drug release enteric formulations (with a suitable enteric coating). Finally, a HPMC/ADCP ratio between 0.11 and 0.69 (H4) yield a gastrointestinal controlled drug release, due to its time-dependent buoyancy (7 h) and a total drug delivery in 17 h in simulated colonic fluid.
Figures

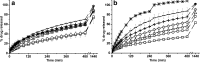

Similar articles
-
Pectin/anhydrous dibasic calcium phosphate matrix tablets for in vitro controlled release of water-soluble drug.Int J Pharm. 2015 Oct 15;494(1):235-43. doi: 10.1016/j.ijpharm.2015.08.027. Epub 2015 Aug 11. Int J Pharm. 2015. PMID: 26276258
-
Effect of hydroxypropyl methylcellulose and hydrogenated castor oil on naproxen release from sustained-release tablets.AAPS PharmSciTech. 2001 Apr 9;2(2):E6. doi: 10.1208/pt020206. AAPS PharmSciTech. 2001. PMID: 14727881 Free PMC article.
-
In Vitro and In Vivo Modeling of Hydroxypropyl Methylcellulose (HPMC) Matrix Tablet Erosion Under Fasting and Postprandial Status.Pharm Res. 2017 Apr;34(4):847-859. doi: 10.1007/s11095-017-2113-7. Epub 2017 Feb 2. Pharm Res. 2017. PMID: 28155077 Free PMC article.
-
Effects of manufacturing process variables on in vitro dissolution characteristics of extended-release tablets formulated with hydroxypropyl methylcellulose.Drug Dev Ind Pharm. 2003 Jan;29(1):79-88. doi: 10.1081/ddc-120016686. Drug Dev Ind Pharm. 2003. PMID: 12602495
-
Release of theophylline and carbamazepine from matrix tablets--consequences of HPMC chemical heterogeneity.Eur J Pharm Biopharm. 2011 Aug;78(3):470-9. doi: 10.1016/j.ejpb.2011.02.003. Epub 2011 Feb 18. Eur J Pharm Biopharm. 2011. PMID: 21316446
Cited by
-
Budding Multi-matrix Technology-a Retrospective Approach, Deep Insights, and Future Perspectives.AAPS PharmSciTech. 2021 Nov 3;22(8):264. doi: 10.1208/s12249-021-02133-4. AAPS PharmSciTech. 2021. PMID: 34734325 Review.
-
Silicon Oxycarbide Porous Particles and Film Coating as Strategies for Tenofovir Controlled Release in Vaginal Tablets for HIV Prevention.Pharmaceutics. 2022 Jul 28;14(8):1567. doi: 10.3390/pharmaceutics14081567. Pharmaceutics. 2022. PMID: 36015193 Free PMC article.
-
Smart Freeze-Dried Bigels for the Prevention of the Sexual Transmission of HIV by Accelerating the Vaginal Release of Tenofovir during Intercourse.Pharmaceutics. 2019 May 13;11(5):232. doi: 10.3390/pharmaceutics11050232. Pharmaceutics. 2019. PMID: 31086015 Free PMC article.
-
Influence of Chitosan Swelling Behaviour on Controlled Release of Tenofovir from Mucoadhesive Vaginal Systems for Prevention of Sexual Transmission of HIV.Mar Drugs. 2017 Feb 21;15(2):50. doi: 10.3390/md15020050. Mar Drugs. 2017. PMID: 28230790 Free PMC article.
-
Virtual Clinical Trials Guided Design of an Age-Appropriate Formulation and Dosing Strategy of Nifedipine for Paediatric Use.Pharmaceutics. 2023 Feb 7;15(2):556. doi: 10.3390/pharmaceutics15020556. Pharmaceutics. 2023. PMID: 36839878 Free PMC article.
References
-
- Sung KC, Nixon PR, Skoug JW, Ju TR, Gao P, Topp EM, Patel MV. Effect of formulation variables on drug and polymer release from HPMC-based matrix tablets. Int J Pharm. 1996;142:53–60. doi: 10.1016/0378-5173(96)04644-3. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

