Schistosoma mansoni infection impairs antimalaria treatment and immune responses of rhesus macaques infected with mosquito-borne Plasmodium coatneyi
- PMID: 22907811
- PMCID: PMC3486041
- DOI: 10.1128/IAI.00590-12
Schistosoma mansoni infection impairs antimalaria treatment and immune responses of rhesus macaques infected with mosquito-borne Plasmodium coatneyi
Abstract
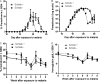
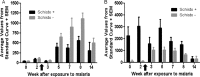
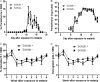
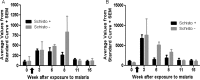
Malaria and schistosomiasis are the world's two most important parasitic infections in terms of distribution, morbidity, and mortality. In areas where Plasmodium and Schistosoma species are both endemic, coinfections are commonplace. Mouse models demonstrate that schistosomiasis worsens a malaria infection; however, just as mice and humans differ greatly, the murine-infecting Plasmodium species differ as much from those that infect humans. Research into human coinfections (Schistosoma haematobium-Plasmodium falciparum versus Schistosoma mansoni-P. falciparum) has produced conflicting results. The rhesus macaque model provides a helpful tool for understanding the role of S. mansoni on malaria parasitemia and antimalarial immune responses using Plasmodium coatneyi, a malaria species that closely resembles P. falciparum infection in humans. Eight rhesus macaques were exposed to S. mansoni cercariae. Eight weeks later, these animals plus 8 additional macaques were exposed to malaria either through bites of infected mosquitos or intravenous inoculation. When malaria infection was initiated from mosquito bites, coinfected animals displayed increased malaria parasitemia, decreased hematocrit levels, and suppressed malaria-specific antibody responses compared to those of malaria infection alone. However, macaques infected by intravenous inoculation with erythrocytic-stage parasites did not display these same differences in parasitemia, hematocrit, or antibody responses between the two groups. Use of the macaque model provides information that begins to unravel differences in pathological and immunological outcomes observed between humans with P. falciparum that are coinfected with S. mansoni or S. haematobium. Our results suggest that migration of malaria parasites through livers harboring schistosome eggs may alter host immune responses and infection outcomes.
Figures
Similar articles
-
Altered immune responses in mice with concomitant Schistosoma mansoni and Plasmodium chabaudi infections.Infect Immun. 1998 Nov;66(11):5167-74. doi: 10.1128/IAI.66.11.5167-5174.1998. Infect Immun. 1998. PMID: 9784518 Free PMC article.
-
Increased parasitaemia and delayed parasite clearance in Schistosoma mansoni and Plasmodium berghei co-infected mice.Acta Trop. 2004 Jul;91(2):161-6. doi: 10.1016/j.actatropica.2004.04.002. Acta Trop. 2004. PMID: 15234665
-
Effect of artemether administered alone or in combination with praziquantel to mice infected with Plasmodium berghei or Schistosoma mansoni or both.Int J Parasitol. 2006 Jul;36(8):957-64. doi: 10.1016/j.ijpara.2006.03.007. Epub 2006 Apr 25. Int J Parasitol. 2006. PMID: 16750833
-
A Review of Plasmodium coatneyi-Macaque Models of Severe Malaria.Vet Pathol. 2015 Nov;52(6):998-1011. doi: 10.1177/0300985815583098. Epub 2015 Jun 15. Vet Pathol. 2015. PMID: 26077782 Review.
-
The role of naturally acquired antimalarial antibodies in subclinical Plasmodium spp. infection.J Leukoc Biol. 2022 May;111(5):1097-1105. doi: 10.1002/JLB.5MR1021-537R. Epub 2022 Jan 20. J Leukoc Biol. 2022. PMID: 35060185 Free PMC article. Review.
Cited by
-
Protective Effect of Chronic Schistosomiasis in Baboons Coinfected with Schistosoma mansoni and Plasmodium knowlesi.Infect Immun. 2016 Apr 22;84(5):1320-1330. doi: 10.1128/IAI.00490-15. Print 2016 May. Infect Immun. 2016. PMID: 26883586 Free PMC article.
-
Non-Human Primate Malaria Infections: A Review on the Epidemiology in Malaysia.Int J Environ Res Public Health. 2022 Jun 27;19(13):7888. doi: 10.3390/ijerph19137888. Int J Environ Res Public Health. 2022. PMID: 35805545 Free PMC article. Review.
-
Coinfection with Schistosoma mansoni Enhances Disease Severity in Human African Trypanosomiasis.J Trop Med. 2023 Nov 3;2023:1063169. doi: 10.1155/2023/1063169. eCollection 2023. J Trop Med. 2023. PMID: 37954132 Free PMC article.
-
Variation in Local and Systemic Pro-Inflammatory Immune Markers of Wild Wood Mice after Anthelmintic Treatment.Integr Comp Biol. 2019 Nov 1;59(5):1190-1202. doi: 10.1093/icb/icz136. Integr Comp Biol. 2019. PMID: 31368489 Free PMC article.
-
In vivo imaging in NHP models of malaria: challenges, progress and outlooks.Parasitol Int. 2014 Feb;63(1):206-15. doi: 10.1016/j.parint.2013.09.001. Epub 2013 Sep 14. Parasitol Int. 2014. PMID: 24042056 Free PMC article. Review.
References
-
- Barnwell JW. 2006. Malaria: death and disappearing erythrocytes. Blood 107:854–855
-
- Briand V, Watier L, Le Hesran JY, Garcia A, Cot M. 2005. Coinfection with Plasmodium falciparum and Schistosoma haematobium: protective effect of schistosomiasis on malaria in Senegalese children? Am. J. Trop. Med. Hyg. 72:702–707 - PubMed
-
- Buck AA, Anderson RI, MacRae AA. 1978. Epidemiology of poly-parasitism. II. Types of combinations, relative frequency and associations of multiple infections. Tropenmed. Parasitol. 29:137–144 - PubMed
-
- Buck AA, Anderson RI, MacRae AA. 1978. Epidemiology of poly-parasitism. III. Effects on the diagnostic capacity of immunological tests. Tropenmed. Parasitol. 29:145–155 - PubMed
-
- Buck AA, Anderson RI, MacRae AA. 1978. Epidemiology of poly-parasitism. IV. Combined effects on the state of health. Tropenmed. Parasitol. 29:253–268 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

