Dysferlin and animal models for dysferlinopathy
- PMID: 22907980
- PMCID: PMC3392904
- DOI: 10.1293/tox.25.135
Dysferlin and animal models for dysferlinopathy
Abstract
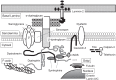
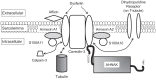
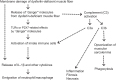
Dysferlin (DYSF) is involved in the membrane-repair process, in the intracellular vesicle system and in T-tubule development in skeletal muscle. It interacts with mitsugumin 53, annexins, caveolin-3, AHNAK, affixin, S100A10, calpain-3, tubulin and dihydropyridine receptor. Limb-girdle muscular dystrophy 2B (LGMD2B) and Miyoshi myopathy (MM) are muscular dystrophies associated with recessively inherited mutations in the DYSF gene. The diseases are characterized by weakness and muscle atrophy that progress slowly and symmetrically in the proximal muscles of the limb girdles. LGMD2B and MM, which are collectively termed "dysferlinopathy", both lead to abnormalities in vesicle traffic and membrane repair at the plasma membrane in muscle fibers. SJL/J (SJL) and A/J mice are naturally occurring animal models for dysferlinopathy. Since there has been no an approach to therapy for dysferlinopathy, the immediate development of a therapeutic method for this genetic disorder is desirable. The murine models are useful in verification experiments for new therapies and they are valuable tools for identifying factors that accelerate dystrophic changes in skeletal muscle. It could be possible that the genetic or immunological background in SJL or A/J mice could modify muscle damage in experiments involving these models, because SJL and A/J mice show differences in the progress and prevalent sites of skeletal muscle lesions as well as in the gene-expression profiles of their skeletal muscle. In this review, we provide up-to-date information on the function of dysferlin, the development of possible therapies for muscle dystrophies (including dysferlinopathy) and the detection of new therapeutic targets for dysferlinopathy by means of experiments using animal models for dysferlinopathy.
Keywords: A/J mouse; SJL/J mouse; complement; dysferlin; dysferlinopathy.
Figures


Similar articles
-
Limb-Girdle Muscular Dystrophy 2B and Miyoshi Presentations of Dysferlinopathy.Am J Med Sci. 2017 May;353(5):484-491. doi: 10.1016/j.amjms.2016.05.024. Epub 2016 May 30. Am J Med Sci. 2017. PMID: 28502335 Review.
-
Dysferlin interacts with calsequestrin-1, myomesin-2 and dynein in human skeletal muscle.Int J Biochem Cell Biol. 2013 Aug;45(8):1927-38. doi: 10.1016/j.biocel.2013.06.007. Epub 2013 Jun 19. Int J Biochem Cell Biol. 2013. PMID: 23792176
-
Dysferlinopathy Fibroblasts Are Defective in Plasma Membrane Repair.PLoS Curr. 2015 Oct 29;7:ecurrents.md.5865add2d766f39a0e0411d38a7ba09c. doi: 10.1371/currents.md.5865add2d766f39a0e0411d38a7ba09c. PLoS Curr. 2015. PMID: 26579332 Free PMC article.
-
The distribution and characterization of skeletal muscle lesions in dysferlin-deficient SJL and A/J mice.Exp Toxicol Pathol. 2010 Sep;62(5):509-17. doi: 10.1016/j.etp.2009.06.009. Epub 2009 Jul 16. Exp Toxicol Pathol. 2010. PMID: 19615872
-
Dysferlinopathy.2004 Feb 5 [updated 2021 May 27]. In: Adam MP, Feldman J, Mirzaa GM, Pagon RA, Wallace SE, Amemiya A, editors. GeneReviews® [Internet]. Seattle (WA): University of Washington, Seattle; 1993–2025. 2004 Feb 5 [updated 2021 May 27]. In: Adam MP, Feldman J, Mirzaa GM, Pagon RA, Wallace SE, Amemiya A, editors. GeneReviews® [Internet]. Seattle (WA): University of Washington, Seattle; 1993–2025. PMID: 20301480 Free Books & Documents. Review.
Cited by
-
Mutation at a new allele of the dysferlin gene causes Miyoshi myopathy: A case report.J Musculoskelet Neuronal Interact. 2021 Sep 1;21(3):397-400. J Musculoskelet Neuronal Interact. 2021. PMID: 34465679 Free PMC article.
-
Structure-Based Designed Nano-Dysferlin Significantly Improves Dysferlinopathy in BLA/J Mice.Mol Ther. 2017 Sep 6;25(9):2150-2162. doi: 10.1016/j.ymthe.2017.05.013. Epub 2017 Jun 16. Mol Ther. 2017. PMID: 28629822 Free PMC article.
-
Increased nonHDL cholesterol levels cause muscle wasting and ambulatory dysfunction in the mouse model of LGMD2B.J Lipid Res. 2018 Feb;59(2):261-272. doi: 10.1194/jlr.M079459. Epub 2017 Nov 25. J Lipid Res. 2018. PMID: 29175948 Free PMC article.
-
Identification of Novel Antisense-Mediated Exon Skipping Targets in DYSF for Therapeutic Treatment of Dysferlinopathy.Mol Ther Nucleic Acids. 2018 Dec 7;13:596-604. doi: 10.1016/j.omtn.2018.10.004. Epub 2018 Oct 11. Mol Ther Nucleic Acids. 2018. PMID: 30439648 Free PMC article.
-
A new dysferlin gene mutation in a Portuguese family with Miyoshi myopathy.BMJ Case Rep. 2021 Jul 19;14(7):e242341. doi: 10.1136/bcr-2021-242341. BMJ Case Rep. 2021. PMID: 34281941 Free PMC article.
References
-
- Cohn RD, Campbell KP. Molecular basis of muscular dystrophies. Muscle Nerve. 23: 1456–1471 2000. - PubMed
-
- Bushby KM. Making sense of the limb-girdle muscular dystrophies. Brain. 122: 1403–1420 1999. - PubMed
-
- Glover L, Brown RH., JrDysferlin in membrane trafficking and patch repair. Traffic. 8: 785–794 2007. - PubMed
-
- Centers for Disease Control and Prevention (CDC) Prevalence of Duchenne/Becker muscular dystrophy among males aged 5–24 years — four states, 2007. Morb Mortal Wkly Rep. 58: 1119–1122 2009 - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous
