Functional Human Vascular Network Generated in Photocrosslinkable Gelatin Methacrylate Hydrogels
- PMID: 22907987
- PMCID: PMC3422083
- DOI: 10.1002/adfm.201101662
Functional Human Vascular Network Generated in Photocrosslinkable Gelatin Methacrylate Hydrogels
Abstract
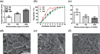
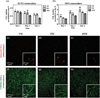
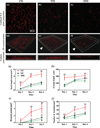
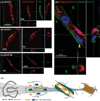
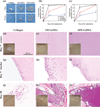
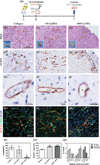
The generation of functional, 3D vascular networks is a fundamental prerequisite for the development of many future tissue engineering-based therapies. Current approaches in vascular network bioengineering are largely carried out using natural hydrogels as embedding scaffolds. However, most natural hydrogels present a poor mechanical stability and a suboptimal durability, which are critical limitations that hamper their widespread applicability. The search for improved hydrogels has become a priority in tissue engineering research. Here, the suitability of a photopolymerizable gelatin methacrylate (GelMA) hydrogel to support human progenitor cell-based formation of vascular networks is demonstrated. Using GelMA as the embedding scaffold, it is shown that 3D constructs containing human blood-derived endothelial colony-forming cells (ECFCs) and bone marrow-derived mesenchymal stem cells (MSCs) generate extensive capillary-like networks in vitro. These vascular structures contain distinct lumens that are formed by the fusion of ECFC intracellular vacuoles in a process of vascular morphogenesis. The process of vascular network formation is dependent on the presence of MSCs, which differentiate into perivascular cells occupying abluminal positions within the network. Importantly, it is shown that implantation of cell-laden GelMA hydrogels into immunodeficient mice results in a rapid formation of functional anastomoses between the bioengineered human vascular network and the mouse vasculature. Furthermore, it is shown that the degree of methacrylation of the GelMA can be used to modulate the cellular behavior and the extent of vascular network formation both in vitro and in vivo. These data suggest that GelMA hydrogels can be used for biomedical applications that require the formation of microvascular networks, including the development of complex engineered tissues.
Figures


References
-
- Levenberg S, Rouwkema J, Macdonald M, Garfein ES, Kohane DS, Darland DC, Marini R, van Blitterswijk CA, Mulligan RC, D’Amore PA, Langer R. Nat. Biotechnol. 2005;23:879. - PubMed
-
- Langer R, Vacanti JP. Science. 1993;260:920. - PubMed
-
- Jain RK, Au P, Tam J, Duda DG, Fukumura D. Nat. Biotechnol. 2005;23:821. - PubMed
-
- Isner JM, Pieczek A, Schainfeld R, Blair R, Haley L, Asahara T, Rosenfield K, Razvi S, Walsh K, Symes JF. Lancet. 1996;348:370. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
