Activation of cortical 5-HT(3) receptor-expressing interneurons induces NO mediated vasodilatations and NPY mediated vasoconstrictions
- PMID: 22907992
- PMCID: PMC3415676
- DOI: 10.3389/fncir.2012.00050
Activation of cortical 5-HT(3) receptor-expressing interneurons induces NO mediated vasodilatations and NPY mediated vasoconstrictions
Abstract
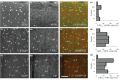
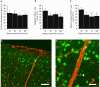
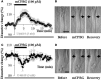
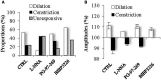
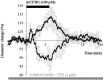
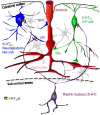
GABAergic interneurons are local integrators of cortical activity that have been reported to be involved in the control of cerebral blood flow (CBF) through their ability to produce vasoactive molecules and their rich innervation of neighboring blood vessels. They form a highly diverse population among which the serotonin 5-hydroxytryptamine 3A receptor (5-HT(3A))-expressing interneurons share a common developmental origin, in addition to the responsiveness to serotonergic ascending pathway. We have recently shown that these neurons regroup two distinct subpopulations within the somatosensory cortex: Neuropeptide Y (NPY)-expressing interneurons, displaying morphological properties similar to those of neurogliaform cells and Vasoactive Intestinal Peptide (VIP)-expressing bipolar/bitufted interneurons. The aim of the present study was to determine the role of these neuronal populations in the control of vascular tone by monitoring blood vessels diameter changes, using infrared videomicroscopy in mouse neocortical slices. Bath applications of 1-(3-Chlorophenyl)biguanide hydrochloride (mCPBG), a 5-HT(3)R agonist, induced both constrictions (30%) and dilations (70%) of penetrating arterioles within supragranular layers. All vasoconstrictions were abolished in the presence of the NPY receptor antagonist (BIBP 3226), suggesting that they were elicited by NPY release. Vasodilations persisted in the presence of the VIP receptor antagonist VPAC1 (PG-97-269), whereas they were blocked in the presence of the neuronal Nitric Oxide (NO) Synthase (nNOS) inhibitor, L-NNA. Altogether, these results strongly suggest that activation of neocortical 5-HT(3A)-expressing interneurons by serotoninergic input could induces NO mediated vasodilatations and NPY mediated vasoconstrictions.
Keywords: Pet1 knock-out mouse; U46619; brain slices; mCPBG; neurogliaform cells; neurovascular coupling; serotonin; vasoactive intestinal peptide.
Figures



References
-
- Abounader R., Elhusseiny A., Cohen Z., Olivier A., Stanimirovic D., Quirion R., Hamel E. (1999). Expression of neuropeptide Y receptors mRNA and protein in human brain vessels and cerebromicrovascular cells in culture. J. Cereb. Blood Flow Metab. 19, 155–163 10.1097/00004647-199902000-00007 - DOI - PubMed
-
- Ascoli G. A., Alonso-Nanclares L., Anderson S. A., Barrionuevo G., Benavides-Piccione R., Burkhalter A., Buzàki G., Cauli B., Defelipe J., Faién A., Feldmeyer D., Fishell G., Fregnac Y., Freund T. F., Gardner E. P., Goldberg J. H., Helmstaedter M., Hestrin S., Karube F., Kisvàrday Z. F., Lambolez B., Lewis D. A., Marin O., Markram H., Munoz A., Packer A., Petersen C. C., Rockland K. S., Rossier J., Rudy B., Somogy P., Staiger J. F., Tamas G., Thomson A. M., Toledo-Rodriguez M., Wang Y., West D. C., Yuste R. (2008). Petilla terminology: nomenclature of features of GABAergic interneurons of the cerebral cortex. Nat. Rev. Neurosci. 9, 557–568 10.1038/nrn2402 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

