Periosteal Sharpey's fibers: a novel bone matrix regulatory system?
- PMID: 22908007
- PMCID: PMC3414712
- DOI: 10.3389/fendo.2012.00098
Periosteal Sharpey's fibers: a novel bone matrix regulatory system?
Abstract
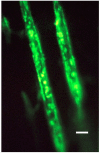
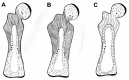
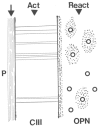
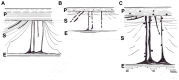
Sharpey's "perforating" fibers (SF) are well known skeletally in tooth anchorage. Elsewhere they provide anchorage for the periosteum and are less well documented. Immunohistochemistry has transformed their potential significance by identifying their collagen type III (CIII) content and enabling their mapping in domains as permeating arrays of fibers (5-25 μ thick), protected from osteoclastic resorption by their poor mineralization. As periosteal extensions they are crucial in early skeletal development and central to intramembranous bone healing, providing unique microanatomical avenues for musculoskeletal exchange, their composition (e.g., collagen type VI, elastin, tenascin) combined with a multiaxial pattern of insertion suggesting a role more complex than attachment alone would justify. A proportion permeate the cortex to the endosteum (and beyond), fusing into a CIII-rich osteoid layer (<2 μ thick) encompassing all resting surfaces, and with which they apparently integrate into a PERIOSTEAL-SHARPEY FIBER-ENDOSTEUM (PSE) structural continuum. This intraosseous system behaves in favor of bone loss or gain depending upon extraneous stimuli (i.e., like Frost's hypothetical "mechanostat"). Thus, the birefringent fibers are sensitive to humoral factors (e.g., estrogen causes retraction, rat femur model), physical activity (e.g., running causes expansion, rat model), aging (e.g., causes fragmentation, pig mandible model), and pathology (e.g., atrophied in osteoporosis, hypertrophied in osteoarthritis, human proximal femur), and with encroaching mineral particles hardening the usually soft parts. In this way the unobtrusive periosteal SF network may regulate bone status, perhaps even contributing to predictable "hotspots" of trabecular disconnection, particularly at sites of tension prone to fatigue, and with the network deteriorating significantly before bone matrix loss.
Keywords: collagen type III; collagen type VI; elastin; endosteal membrane; matrix biochemical domains; skeletal aging; tenascin.
Figures


References
-
- Aaron J. E. (1977). Autoclasis – a mechanism of bone resorption and an alternative explanation for osteoporosis. Calcif. Tissue Res. 22 S247–S254 - PubMed
-
- Aaron J. E. (1980a). Demineralization of bone in vivo and in vitro. Metab. Bone Dis. Relat. Res. 2S 117–125
-
- Aaron J. E. (1980b). Alkaline phosphatase, vesicles and calcification. Metab. Bone Dis. Relat. Res. 2S 151–157
-
- Aaron J. E. (2003). Bone turnover and microdamage. Adv. Osteoporotic Fract. Manag. 2 102–110
-
- Aaron J. E., Carter D. H. (1987). Rapid preparation of fresh frozen undecalcified bone for histology and histochemical analysis. J. Histochem. Cytochem. 35 361–369 - PubMed
LinkOut - more resources
Full Text Sources
Research Materials

