The challenges of determining noninferiority margins: a case study of noninferiority randomized controlled trials of novel oral anticoagulants
- PMID: 22908144
- PMCID: PMC3576440
- DOI: 10.1503/cmaj.120142
The challenges of determining noninferiority margins: a case study of noninferiority randomized controlled trials of novel oral anticoagulants
Figures

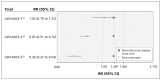
References
-
- Wangge G, Klungel OH, Roes KC, et al. Interpretation and inference in noninferiority randomized controlled trials in drug research. Clin Pharmacol Ther 2010. ;88:420–3 - PubMed
-
- Guidance for industry non-inferiority clinical trials. Silver Spring (MD): Center for Drug Evaluation and Research; and Rockville (MD): Center for Biologics Evaluation and Research, US Food and Drug Administration; 2010. Available: www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guid... (accessed 2012 June 15).
-
- ICH Expert Working Group ICH Harmonised Tripartite Guideline: Statistical principles for clinical trials. E9 Geneva: ICH; 1998. Available: www.ich.org/fileadmin/Public_Web_Site/ICH_Products/Guidelines/Efficacy/E... (accessed 2012 June 15). - PubMed
-
- ICH Expert Working Group ICH Harmonised Tripartite Guideline: Choice of control group and related issues in clinical trials. E-10 Geneva: ICH; 2000. Available: www.ich.org/fileadmin/Public_Web_Site/ICH_Products/Guidelines/Efficacy/E... (accessed 2012 June 15).
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
