Toxicity mechanisms of amphotericin B and its neutralization by conjugation with arabinogalactan
- PMID: 22908154
- PMCID: PMC3486601
- DOI: 10.1128/AAC.00612-12
Toxicity mechanisms of amphotericin B and its neutralization by conjugation with arabinogalactan
Abstract
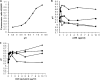
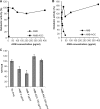
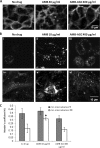
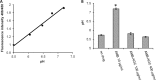
Amphotericin B (AMB) is an effective antifungal agent. However, its therapeutic use is hampered by its toxicity, mainly due to channel formation across kidney cell membranes and the disruption of postendocytic trafficking. We previously described a safe injectable AMB-arabinogalactan (AG) conjugate with neutralized toxicity. Here we studied the mechanism of the toxicity of free AMB and its neutralization by conjugation with AG. AMB treatment of a kidney cell line modulated the trafficking of three receptors (C-X-C chemokine receptor type 4 [CXCR4], M1 receptor, and human transferrin receptor [hTfnR]) due to an increase in endosomal pH. Similar data were also obtained in yeast but with an increase in vacuolar pH and the perturbation of Hxt2-green fluorescent protein (GFP) trafficking. The conjugation of AMB with AG neutralized all elements of the toxic activity of AMB in mammalian but not in fungal cells. Based on these results, we provide an explanation of how the conjugation of AMB with AG neutralizes its toxicity in mammalian cells and add to the knowledge of the mechanism of action of free AMB in both fungal and mammalian cells.
Figures

References
-
- Ali R, Brett CL, Mukherjee S, Rao R. 2004. Inhibition of sodium/proton exchange by a Rab-GTPase-activating protein regulates endosomal traffic in yeast. J. Biol. Chem. 279:4498–4506 - PubMed
-
- Arnold TM, Dotson E, Sarosi GA, Hage CA. 2010. Traditional and emerging antifungal therapies. Proc. Am. Thorac. Soc. 7:222–228 - PubMed
-
- Baginski M, Resat H, Borowski E. 2002. Comparative molecular dynamics simulations of amphotericin B-cholesterol/ergosterol membrane channels. Biochim. Biophys. Acta 1567:63–78 - PubMed
-
- Bolard J. 1986. How do the polyene macrolide antibiotics affect the cellular membrane properties? Biochim. Biophys. Acta 864:257–304 - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

