Population pharmacokinetics of piperacillin at two dose levels: influence of nonlinear pharmacokinetics on the pharmacodynamic profile
- PMID: 22908169
- PMCID: PMC3486597
- DOI: 10.1128/AAC.00937-12
Population pharmacokinetics of piperacillin at two dose levels: influence of nonlinear pharmacokinetics on the pharmacodynamic profile
Abstract
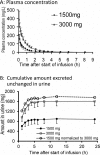
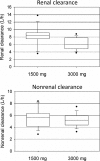
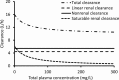
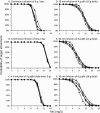
Piperacillin in combination with tazobactam is one of the most commonly used intravenous antibiotics. There is evidence for a possible saturable elimination of piperacillin. Therefore, the saturable elimination and its impact on the choice of optimal dosage regimens were quantified. In a randomized crossover study, 10 healthy volunteers received 1,500 mg and 3,000 mg of piperacillin as 5-min intravenous infusion. Population pharmacokinetics based on plasma and urine data were determined utilizing NONMEM and S-ADAPT. Probabilities of target attainment (PTAs) were compared for different models and dosage regimens, based on the target time of the non-protein-bound concentration above the MIC of at least 50% of the dosing interval. Total clearance of piperacillin was 18% (geometric mean ratio, 90% confidence interval, 11 to 24%) lower (P < 0.01), and renal clearance was 24% (9 to 37%) lower (P = 0.02) at the high compared to the low dose. The final model included first-order nonrenal elimination and parallel first-order and mixed-order renal elimination. Nonrenal clearance was 5.44 liter/h (coefficient of variation, 18%), first-order renal clearance was 4.42 liter/h (47%), and the maximum elimination rate of mixed-order renal elimination was 219 mg/h (84%), with a Michaelis-Menten constant of 36.1 mg/liter (112%). Compared to models with saturable elimination, a linear model predicted up to 10% lower population PTAs for high-dose short-term infusions (6 g every 8 h) and up to 4% higher population PTAs for low-dose continuous infusions (6 g/day). While renal elimination of piperacillin was saturable at therapeutic concentrations, the extent of saturation of nonrenal clearance was small. The influence of saturable elimination on PTAs for clinically relevant dosage regimens was relatively small.
Figures

References
-
- Ambrose PG, Bhavnani SM, Jones RN. 2003. Pharmacokinetics-pharmacodynamics of cefepime and piperacillin-tazobactam against Escherichia coli and Klebsiella pneumoniae strains producing extended-spectrum beta-lactamases: report from the ARREST program. Antimicrob. Agents Chemother. 47:1643–1646 - PMC - PubMed
-
- Ambrose PG, et al. 2007. Pharmacokinetics-pharmacodynamics of antimicrobial therapy: it's not just for mice anymore. Clin. Infect. Dis. 44:79–86 - PubMed
-
- Andes D, Craig WA. 2002. Animal model pharmacokinetics and pharmacodynamics: a critical review. Int. J. Antimicrob. Agents 19:261–268 - PubMed
-
- Anonymous 1999. Piperacillin sodium and tazobactam sodium (Zosyn) product information. Lederle Laboratories, Pearl River, NY
-
- Aronoff GR, Sloan RS, Brier ME, Luft FC. 1983. The effect of piperacillin dose on elimination kinetics in renal impairment. Eur. J. Clin. Pharmacol. 24:543–547 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

