PlGF: a multitasking cytokine with disease-restricted activity
- PMID: 22908198
- PMCID: PMC3405829
- DOI: 10.1101/cshperspect.a011056
PlGF: a multitasking cytokine with disease-restricted activity
Abstract
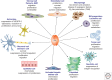
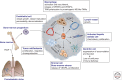
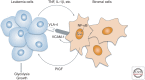
Placental growth factor (PlGF) is a member of the vascular endothelial growth factor (VEGF) family that also comprises VEGF-A (VEGF), VEGF-B, VEGF-C, and VEGF-D. Unlike VEGF, PlGF is dispensable for development and health but has diverse nonredundant roles in tissue ischemia, malignancy, inflammation, and multiple other diseases. Genetic and pharmacological gain-of-function and loss-of-function studies have identified molecular mechanisms of this multitasking cytokine and characterized the therapeutic potential of delivering or blocking PlGF for various disorders.
Figures

Similar articles
-
Synergism between vascular endothelial growth factor and placental growth factor contributes to angiogenesis and plasma extravasation in pathological conditions.Nat Med. 2001 May;7(5):575-83. doi: 10.1038/87904. Nat Med. 2001. PMID: 11329059
-
Structure and function of placental growth factor.Trends Cardiovasc Med. 2002 Aug;12(6):241-6. doi: 10.1016/s1050-1738(02)00168-8. Trends Cardiovasc Med. 2002. PMID: 12242046 Review.
-
Prevention of elastase-induced emphysema in placenta growth factor knock-out mice.Respir Res. 2009 Nov 23;10(1):115. doi: 10.1186/1465-9921-10-115. Respir Res. 2009. PMID: 19930612 Free PMC article.
-
Placental growth factor and its receptor, vascular endothelial growth factor receptor-1: novel targets for stimulation of ischemic tissue revascularization and inhibition of angiogenic and inflammatory disorders.J Thromb Haemost. 2003 Jul;1(7):1356-70. doi: 10.1046/j.1538-7836.2003.00263.x. J Thromb Haemost. 2003. PMID: 12871269 Review.
-
Loss of placental growth factor protects mice against vascular permeability in pathological conditions.Biochem Biophys Res Commun. 2002 Jul 12;295(2):428-34. doi: 10.1016/s0006-291x(02)00677-0. Biochem Biophys Res Commun. 2002. PMID: 12150967
Cited by
-
PlGF and VEGF-A Regulate Growth of High-Risk MYCN-Single Copy Neuroblastoma Xenografts via Different Mechanisms.Int J Mol Sci. 2016 Sep 23;17(10):1613. doi: 10.3390/ijms17101613. Int J Mol Sci. 2016. PMID: 27669225 Free PMC article.
-
Recent molecular discoveries in angiogenesis and antiangiogenic therapies in cancer.J Clin Invest. 2013 Aug;123(8):3190-200. doi: 10.1172/JCI70212. Epub 2013 Aug 1. J Clin Invest. 2013. PMID: 23908119 Free PMC article. Review.
-
Interdependence of Angiogenesis and Arteriogenesis in Development and Disease.Int J Mol Sci. 2022 Mar 31;23(7):3879. doi: 10.3390/ijms23073879. Int J Mol Sci. 2022. PMID: 35409246 Free PMC article. Review.
-
Epigenetic control of hypoxia inducible factor-1α-dependent expression of placental growth factor in hypoxic conditions.Epigenetics. 2014 Apr;9(4):600-10. doi: 10.4161/epi.27835. Epub 2014 Feb 6. Epigenetics. 2014. PMID: 24504136 Free PMC article.
-
Elastase induced lung epithelial cell apoptosis and emphysema through placenta growth factor.Cell Death Dis. 2013 Sep 5;4(9):e793. doi: 10.1038/cddis.2013.329. Cell Death Dis. 2013. PMID: 24008737 Free PMC article.
References
-
- Adini A, Kornaga T, Firoozbakht F, Benjamin LE 2002. Placental growth factor is a survival factor for tumor endothelial cells and macrophages. Cancer Res 62: 2749–2752 - PubMed
-
- Akrami H, Soheili ZS, Sadeghizadeh M, Ahmadieh H, Rezaeikanavi M, Samiei S, Khalooghi K 2011. PlGF gene knockdown in human retinal pigment epithelial cells. Graefes Arch Clin Exp Ophthalmol 249: 537–546 - PubMed
-
- Apple FS, Pearce LA, Chung A, Ler R, Murakami MM 2007. Multiple biomarker use for detection of adverse events in patients presenting with symptoms suggestive of acute coronary syndrome. Clin Chem 53: 874–881 - PubMed
-
- Autiero M, Waltenberger J, Communi D, Kranz A, Moons L, Lambrechts D, Kroll J, Plaisance S, De Mol M, Bono F, et al. 2003. Role of PlGF in the intra- and intermolecular cross talk between the VEGF receptors Flt1 and Flk1. Nat Med 9: 936–943 - PubMed
-
- Babiak A, Schumm AM, Wangler C, Loukas M, Wu J, Dombrowski S, Matuschek C, Kotzerke J, Dehio C, Waltenberger J 2004. Coordinated activation of VEGFR-1 and VEGFR-2 is a potent arteriogenic stimulus leading to enhancement of regional perfusion. Cardiovasc Res 61: 789–795 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
