Tetraspanin 6 (TSPAN6) negatively regulates retinoic acid-inducible gene I-like receptor-mediated immune signaling in a ubiquitination-dependent manner
- PMID: 22908223
- PMCID: PMC3464568
- DOI: 10.1074/jbc.M112.390401
Tetraspanin 6 (TSPAN6) negatively regulates retinoic acid-inducible gene I-like receptor-mediated immune signaling in a ubiquitination-dependent manner
Abstract
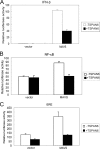
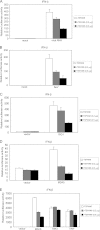
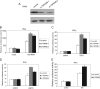
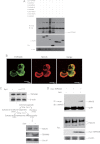
The recognition between retinoic acid-inducible gene I-like receptors (RLRs) and viral RNA triggers an intracellular cascade of signaling to induce the expression of type I IFNs. Both positive and negative regulation of the RLR signaling pathway are important for the host antiviral immune response. Here, we demonstrate that the tetraspanin protein TSPAN6 inhibits RLR signaling by affecting the formation of the adaptor MAVS (mitochondrial antiviral signaling)-centered signalosome. We found that overexpression of TSPAN6 impaired RLR-mediated activation of IFN-stimulated response element, NF-κB, and IFN-β promoters, whereas knockdown of TSPAN6 enhanced the RLR-mediated signaling pathway. Interestingly, as the RLR pathway was activated, TSPAN6 underwent Lys-63-linked ubiquitination, which promoted its association with MAVS. The interaction of TSPAN6 and MAVS interfered with the recruitment of RLR downstream molecules TRAF3, MITA, and IRF3 to MAVS. Further study revealed that the first transmembrane domain of TSPAN6 is critical for its ubiquitination and association with MAVS as well as its inhibitory effect on RLR signaling. We concluded that TSPAN6 functions as a negative regulator of the RLR pathway by interacting with MAVS in a ubiquitination-dependent manner.
Figures



Similar articles
-
DDX3 directly regulates TRAF3 ubiquitination and acts as a scaffold to co-ordinate assembly of signalling complexes downstream from MAVS.Biochem J. 2017 Feb 15;474(4):571-587. doi: 10.1042/BCJ20160956. Epub 2016 Dec 15. Biochem J. 2017. PMID: 27980081
-
HSCARG negatively regulates the cellular antiviral RIG-I like receptor signaling pathway by inhibiting TRAF3 ubiquitination via recruiting OTUB1.PLoS Pathog. 2014 Apr 24;10(4):e1004041. doi: 10.1371/journal.ppat.1004041. eCollection 2014 Apr. PLoS Pathog. 2014. PMID: 24763515 Free PMC article.
-
Functional characterization of domains of IPS-1 using an inducible oligomerization system.PLoS One. 2013;8(1):e53578. doi: 10.1371/journal.pone.0053578. Epub 2013 Jan 7. PLoS One. 2013. PMID: 23308256 Free PMC article.
-
Regulation of RLR-mediated innate immune signaling--it is all about keeping the balance.Eur J Cell Biol. 2012 Jan;91(1):36-47. doi: 10.1016/j.ejcb.2011.01.011. Epub 2011 Apr 9. Eur J Cell Biol. 2012. PMID: 21481967 Review.
-
Antiviral signaling through retinoic acid-inducible gene-I-like receptors.Arch Immunol Ther Exp (Warsz). 2011 Feb;59(1):41-8. doi: 10.1007/s00005-010-0107-9. Epub 2011 Jan 14. Arch Immunol Ther Exp (Warsz). 2011. PMID: 21234810 Review.
Cited by
-
Analysis of Time-Series Gene Expression Data to Explore Mechanisms of Chemical-Induced Hepatic Steatosis Toxicity.Front Genet. 2018 Sep 18;9:396. doi: 10.3389/fgene.2018.00396. eCollection 2018. Front Genet. 2018. PMID: 30279702 Free PMC article.
-
X-linked genes exhibit miR6891-5p-regulated skewing in Sjögren's syndrome.J Mol Med (Berl). 2022 Sep;100(9):1253-1265. doi: 10.1007/s00109-022-02205-3. Epub 2022 May 10. J Mol Med (Berl). 2022. PMID: 35538149 Free PMC article.
-
ALIX- and ESCRT-III-dependent sorting of tetraspanins to exosomes.J Cell Biol. 2020 Mar 2;219(3):e201904113. doi: 10.1083/jcb.201904113. J Cell Biol. 2020. PMID: 32049272 Free PMC article.
-
Negative regulators of the RIG-I-like receptor signaling pathway.Eur J Immunol. 2017 Apr;47(4):615-628. doi: 10.1002/eji.201646484. Eur J Immunol. 2017. PMID: 28295214 Free PMC article. Review.
-
Expression Profiling Analysis Reveals Key MicroRNA-mRNA Interactions in Early Retinal Degeneration in Retinitis Pigmentosa.Invest Ophthalmol Vis Sci. 2018 May 1;59(6):2381-2392. doi: 10.1167/iovs.18-24091. Invest Ophthalmol Vis Sci. 2018. PMID: 29847644 Free PMC article.
References
-
- Ding S. W. (2010) RNA-based antiviral immunity. Nat. Rev. Immunol. 10, 632–644 - PubMed
-
- Meylan E., Curran J., Hofmann K., Moradpour D., Binder M., Bartenschlager R., Tschopp J. (2005) Cardif is an adaptor protein in the RIG-I antiviral pathway and is targeted by hepatitis C virus. Nature 437, 1167–1172 - PubMed
-
- Seth R. B., Sun L., Ea C. K., Chen Z. J. (2005) Identification and characterization of MAVS, a mitochondrial antiviral signaling protein that activates NF-κB and IRF3. Cell 122, 669–682 - PubMed
-
- Kawai T., Takahashi K., Sato S., Coban C., Kumar H., Kato H., Ishii K. J., Takeuchi O., Akira S. (2005) IPS-1, an adaptor triggering RIG-I- and Mda5-mediated type I interferon induction. Nat. Immunol. 6, 981–988 - PubMed
-
- Xu L. G., Wang Y. Y., Han K. J., Li L. Y., Zhai Z., Shu H. B. (2005) VISA is an adaptor protein required for virus-triggered IFN-β signaling. Mol. Cell 19, 727–740 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

