Crystal structure of reaction intermediates in pyruvate class II aldolase: substrate cleavage, enolate stabilization, and substrate specificity
- PMID: 22908224
- PMCID: PMC3476288
- DOI: 10.1074/jbc.M112.400705
Crystal structure of reaction intermediates in pyruvate class II aldolase: substrate cleavage, enolate stabilization, and substrate specificity
Abstract
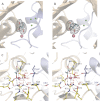
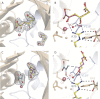
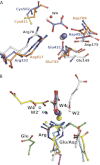
Crystal structures of divalent metal-dependent pyruvate aldolase, HpaI, in complex with substrate and cleavage products were determined to 1.8-2.0 Å resolution. The enzyme·substrate complex with 4-hydroxy-2-ketoheptane-1,7-dioate indicates that water molecule W2 bound to the divalent metal ion initiates C3-C4 bond cleavage. The binding mode of the aldehyde donor delineated a solvent-filled capacious binding locus lined with predominantly hydrophobic residues. The absence of direct interactions with the aldehyde aliphatic carbons accounts for the broad specificity and lack of stereospecific control by the enzyme. Enzymatic complex structures formed with keto acceptors, pyruvate, and 2-ketobutyrate revealed bidentate interaction with the divalent metal ion by C1-carboxyl and C2-carbonyl oxygens and water molecule W4 that is within close contact of the C3 carbon. Arg(70) assumes a multivalent role through its guanidinium moiety interacting with all active site enzymatic species: C2 oxygen in substrate, pyruvate, and ketobutyrate; substrate C4 hydroxyl; aldehyde C1 oxygen; and W4. The multiple interactions made by Arg(70) stabilize the negatively charged C4 oxygen following proton abstraction, the aldehyde alignment in aldol condensation, and the pyruvate enolate upon aldol cleavage as well as support proton exchange at C3. This role is corroborated by loss of aldol cleavage ability and pyruvate C3 proton exchange activity and by a 730-fold increase in the dissociation constant toward the pyruvate enolate analog oxalate in the R70A mutant. Based on the crystal structures, a mechanism is proposed involving the two enzyme-bound water molecules, W2 and W4, in acid/base catalysis that facilitates reversible aldol cleavage. The same reaction mechanism promotes decarboxylation of oxaloacetate.
Figures






Similar articles
-
Purification and biochemical characterization of a pyruvate-specific class II aldolase, HpaI.Biochemistry. 2005 Jul 12;44(27):9447-55. doi: 10.1021/bi050607y. Biochemistry. 2005. PMID: 15996099
-
The role of a conserved histidine residue in a pyruvate-specific Class II aldolase.FEBS Lett. 2008 Oct 15;582(23-24):3385-8. doi: 10.1016/j.febslet.2008.08.032. Epub 2008 Sep 5. FEBS Lett. 2008. PMID: 18775708
-
Structural and kinetic characterization of 4-hydroxy-4-methyl-2-oxoglutarate/4-carboxy-4-hydroxy-2-oxoadipate aldolase, a protocatechuate degradation enzyme evolutionarily convergent with the HpaI and DmpG pyruvate aldolases.J Biol Chem. 2010 Nov 19;285(47):36608-15. doi: 10.1074/jbc.M110.159509. Epub 2010 Sep 15. J Biol Chem. 2010. PMID: 20843800 Free PMC article.
-
Mechanism of the Class I KDPG aldolase.Bioorg Med Chem. 2006 May 1;14(9):3002-10. doi: 10.1016/j.bmc.2005.12.022. Epub 2006 Jan 5. Bioorg Med Chem. 2006. PMID: 16403639 Free PMC article.
-
Sweet siblings with different faces: the mechanisms of FBP and F6P aldolase, transaldolase, transketolase and phosphoketolase revisited in light of recent structural data.Bioorg Chem. 2014 Dec;57:263-280. doi: 10.1016/j.bioorg.2014.09.001. Epub 2014 Sep 16. Bioorg Chem. 2014. PMID: 25267444 Review.
Cited by
-
DHAP-dependent aldolases from (hyper)thermophiles: biochemistry and applications.Extremophiles. 2014 Jan;18(1):1-13. doi: 10.1007/s00792-013-0593-x. Epub 2013 Oct 29. Extremophiles. 2014. PMID: 24166576 Review.
-
Chemoenzymatic Platform for Synthesis of Chiral Organofluorines Based on Type II Aldolases.Angew Chem Int Ed Engl. 2019 Aug 19;58(34):11841-11845. doi: 10.1002/anie.201906805. Epub 2019 Jul 19. Angew Chem Int Ed Engl. 2019. PMID: 31240790 Free PMC article.
-
Genetic code expansion reveals site-specific lactylation in living cells reshapes protein functions.Nat Commun. 2025 Jan 8;16(1):227. doi: 10.1038/s41467-024-55165-2. Nat Commun. 2025. PMID: 39779673 Free PMC article.
-
Amino Acylguanidines as Bioinspired Catalysts for the Asymmetric Aldol Reaction.Molecules. 2021 Feb 5;26(4):826. doi: 10.3390/molecules26040826. Molecules. 2021. PMID: 33562560 Free PMC article.
-
Enhance the performance of current scoring functions with the aid of 3D protein-ligand interaction fingerprints.BMC Bioinformatics. 2017 Jul 18;18(1):343. doi: 10.1186/s12859-017-1750-5. BMC Bioinformatics. 2017. PMID: 28720122 Free PMC article.
References
-
- Takayama S., McGarvey G. J., Wong C. H. (1997) Microbial aldolases and transketolases: new biocatalytic approaches to simple and complex sugars. Annu. Rev. Microbiol. 51, 285–310 - PubMed
-
- Samland A. K., Sprenger G. A. (2006) Microbial aldolases as C–C bonding enzymes—unknown treasures and new developments. Appl. Microbiol. Biotechnol. 71, 253–264 - PubMed
-
- Joerger A. C., Gosse C., Fessner W. D., Schulz G. E. (2000) Catalytic action of fuculose 1-phosphate aldolase (class II) as, derived from structure-directed mutagenesis. Biochemistry 39, 6033–6041 - PubMed
-
- Kroemer M., Merkel I., Schulz G. E. (2003) Structure and catalytic mechanism of l-rhamnulose-1-phosphate aldolase. Biochemistry 42, 10560–10568 - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

