Estrogen represses hepatocellular carcinoma (HCC) growth via inhibiting alternative activation of tumor-associated macrophages (TAMs)
- PMID: 22908233
- PMCID: PMC3504728
- DOI: 10.1074/jbc.M112.348763
Estrogen represses hepatocellular carcinoma (HCC) growth via inhibiting alternative activation of tumor-associated macrophages (TAMs)
Abstract
Background: Hepatocarcinoma cancer (HCC) occurs more often in men than in women, and little is known about its underlying molecular mechanisms.
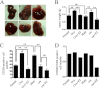
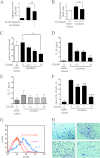
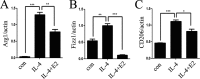
Results: We identify that 17β-estradiol (E2) could suppress tumor growth via regulating the polarization of macrophages.
Conclusion: Estrogen functions as a suppressor for macrophage alternative activation.
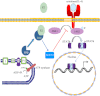
Significance: These studies introduce a novel mechanism for suppressing male-predominant HCC. Hepatocarcinoma cancer (HCC), one of the most malignant cancers, occurs significantly more often in men than in women; however, little is known about its underlying molecular mechanisms. Here we identified that 17β-estradiol (E2) could suppress tumor growth via regulating the polarization of macrophages. We showed that E2 re-administration reduced tumor growth in orthotopic and ectopic mice HCC models. E2 functioned as a suppressor for macrophage alternative activation and tumor progression by keeping estrogen receptor β (ERβ) away from interacting with ATP5J (also known as ATPase-coupling factor 6), a part of ATPase, thus inhibiting the JAK1-STAT6 signaling pathway. These studies introduce a novel mechanism for suppressing male-predominant HCC.
Figures



References
-
- Thomas M. B., Jaffe D., Choti M. M., Belghiti J., Curley S., Fong Y., Gores G., Kerlan R., Merle P., O'Neil B., Poon R., Schwartz L., Tepper J., Yao F., Haller D., Mooney M., Venook A. (2010) Hepatocellular carcinoma. Consensus recommendations of the National Cancer Institute Clinical Trials Planning Meeting. J. Clin. Oncol. 28, 3994–4005 - PMC - PubMed
-
- Rogers A. B., Theve E. J., Feng Y., Fry R. C., Taghizadeh K., Clapp K. M., Boussahmain C., Cormier K. S., Fox J. G. (2007) Hepatocellular carcinoma associated with liver-gender disruption in male mice. Cancer Res. 67, 11536–11546 - PubMed
-
- Ghebranious N., Sell S. (1998) Hepatitis B injury, male gender, aflatoxin, and p53 expression each contribute to hepatocarcinogenesis in transgenic mice. Hepatology 27, 383–391 - PubMed
-
- Maeda S., Kamata H., Luo J. L., Leffert H., Karin M. (2005) IKKβ couples hepatocyte death to cytokine-driven compensatory proliferation that promotes chemical hepatocarcinogenesis. Cell 121, 977–990 - PubMed
-
- Bosch F. X., Ribes J., Díaz M., Cléries R. (2004) Primary liver cancer. Worldwide incidence and trends. Gastroenterology 127, S5–S16 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

