Third target of rapamycin complex negatively regulates development of quiescence in Trypanosoma brucei
- PMID: 22908264
- PMCID: PMC3437835
- DOI: 10.1073/pnas.1210465109
Third target of rapamycin complex negatively regulates development of quiescence in Trypanosoma brucei
Abstract
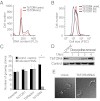
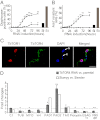
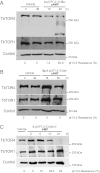
African trypanosomes are protozoan parasites transmitted by a tsetse fly vector to a mammalian host. The life cycle includes highly proliferative forms and quiescent forms, the latter being adapted to host transmission. The signaling pathways controlling the developmental switch between the two forms remain unknown. Trypanosoma brucei contains two target of rapamycin (TOR) kinases, TbTOR1 and TbTOR2, and two TOR complexes, TbTORC1 and TbTORC2. Surprisingly, two additional TOR kinases are encoded in the T. brucei genome. We report that TbTOR4 associates with an Armadillo domain-containing protein (TbArmtor), a major vault protein, and LST8 to form a unique TOR complex, TbTORC4. Depletion of TbTOR4 caused irreversible differentiation of the parasite into the quiescent form. AMP and hydrolysable analogs of cAMP inhibited TbTOR4 expression and induced the stumpy quiescent form. Our results reveal unexpected complexity in TOR signaling and show that TbTORC4 negatively regulates differentiation of the proliferative form into the quiescent form.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Turner CM, Aslam N, Dye C. Replication, differentiation, growth and the virulence of Trypanosoma brucei infections. Parasitology. 1995;111:289–300. - PubMed
-
- Matthews KR, Gull K. Commitment to differentiation and cell cycle re-entry are coincident but separable events in the transformation of African trypanosomes from their bloodstream to their insect form. J Cell Sci. 1997;110:2609–2618. - PubMed
-
- Soulard A, Cohen A, Hall MN. TOR signaling in invertebrates. Curr Opin Cell Biol. 2009;21:825–836. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

