Nonviral delivery of self-amplifying RNA vaccines
- PMID: 22908294
- PMCID: PMC3437863
- DOI: 10.1073/pnas.1209367109
Nonviral delivery of self-amplifying RNA vaccines
Abstract
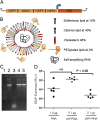
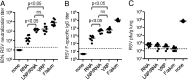
Despite more than two decades of research and development on nucleic acid vaccines, there is still no commercial product for human use. Taking advantage of the recent innovations in systemic delivery of short interfering RNA (siRNA) using lipid nanoparticles (LNPs), we developed a self-amplifying RNA vaccine. Here we show that nonviral delivery of a 9-kb self-amplifying RNA encapsulated within an LNP substantially increased immunogenicity compared with delivery of unformulated RNA. This unique vaccine technology was found to elicit broad, potent, and protective immune responses, that were comparable to a viral delivery technology, but without the inherent limitations of viral vectors. Given the many positive attributes of nucleic acid vaccines, our results suggest that a comprehensive evaluation of nonviral technologies to deliver self-amplifying RNA vaccines is warranted.
Conflict of interest statement
Conflict of interest statement: All authors are Novartis shareholders and employees of Novartis Vaccines and Diagnostics and Novartis Institutes for BioMedical Research.
Figures



Comment in
-
Vaccines: Self-amplifying RNA in lipid nanoparticles: a next-generation vaccine?Nat Rev Drug Discov. 2012 Oct;11(10):748-9. doi: 10.1038/nrd3854. Nat Rev Drug Discov. 2012. PMID: 23023675 No abstract available.
-
Lipid nanoparticle-based delivery of RNA.Nanomedicine (Lond). 2013 Jun;8(6):872-3. Nanomedicine (Lond). 2013. PMID: 23878858 No abstract available.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

