The nerve of ovulation-inducing factor in semen
- PMID: 22908303
- PMCID: PMC3443178
- DOI: 10.1073/pnas.1206273109
The nerve of ovulation-inducing factor in semen
Abstract
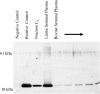
A component in seminal fluid elicits an ovulatory response and has been discovered in every species examined thus far. The existence of an ovulation-inducing factor (OIF) in seminal plasma has broad implications and evokes questions about identity, tissue sources, mechanism of action, role among species, and clinical relevance in infertility. Most of these questions remain unanswered. The goal of this study was to determine the identity of OIF in support of the hypothesis that it is a single distinct and widely conserved entity. Seminal plasma from llamas and bulls was used as representative of induced and spontaneous ovulators, respectively. A fraction isolated from llama seminal plasma by column chromatography was identified as OIF by eliciting luteinizing hormone (LH) release and ovulation in llamas. MALDI-TOF revealed a molecular mass of 13,221 Da, and 12-23 aa sequences of OIF had homology with human, porcine, bovine, and murine sequences of β nerve growth factor (β-NGF). X-ray diffraction data were used to solve the full sequence and structure of OIF as β-NGF. Neurite development and up-regulation of trkA in phaeochromocytoma (PC(12)) cells in vitro confirmed NGF-like properties of OIF. Western blot analysis of llama and bull seminal plasma confirmed immunorecognition of OIF using polyclonal mouse anti-NGF, and administration of β-NGF from mouse submandibular glands induced ovulation in llamas. We conclude that OIF in seminal plasma is β-NGF and that it is highly conserved. An endocrine route of action of NGF elucidates a previously unknown pathway for the direct influence of the male on the hypothalamo-pituitary-gonadal axis of the inseminated female.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
-
- Mann T. Biochemistry of Semen and of the Male Reproductive Tract. Frome, UK: Butler, Tanner Ltd; 1964. p. 493.
-
- Adams GP, Ratto MH, Huanca W, Singh J. Ovulation-inducing factor in the seminal plasma of alpacas and llamas. Biol Reprod. 2005;73:452–457. - PubMed
-
- Bedford JM. Enigmas of mammalian gamete form and function. Biol Rev Camb Philos Soc. 2004;79:429–460. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

