Polo-like kinase-1 regulates kinetochore-microtubule dynamics and spindle checkpoint silencing
- PMID: 22908307
- PMCID: PMC3514039
- DOI: 10.1083/jcb.201205090
Polo-like kinase-1 regulates kinetochore-microtubule dynamics and spindle checkpoint silencing
Abstract
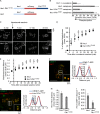
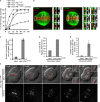
Polo-like kinase-1 (Plk1) is a highly conserved kinase with multiple mitotic functions. Plk1 localizes to prometaphase kinetochores and is reduced at metaphase kinetochores, similar to many checkpoint signaling proteins, but Plk1 is not required for spindle checkpoint function. Plk1 is also implicated in stabilizing kinetochore-microtubule attachments, but these attachments are most stable when kinetochore Plk1 levels are low at metaphase. Therefore, it is unclear how Plk1 function at kinetochores can be understood in the context of its dynamic localization. In this paper, we show that Plk1 activity suppresses kinetochore-microtubule dynamics to stabilize initial attachments in prometaphase, and Plk1 removal from kinetochores is necessary to maintain dynamic microtubules in metaphase. Constitutively targeting Plk1 to kinetochores maintained high activity at metaphase, leading to reduced interkinetochore tension and intrakinetochore stretch, a checkpoint-dependent mitotic arrest, and accumulation of microtubule attachment errors. Together, our data show that Plk1 dynamics at kinetochores control two critical mitotic processes: initially establishing correct kinetochore-microtubule attachments and subsequently silencing the spindle checkpoint.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

