Rab10 and myosin-Va mediate insulin-stimulated GLUT4 storage vesicle translocation in adipocytes
- PMID: 22908308
- PMCID: PMC3514028
- DOI: 10.1083/jcb.201111091
Rab10 and myosin-Va mediate insulin-stimulated GLUT4 storage vesicle translocation in adipocytes
Abstract
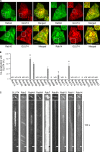
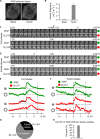
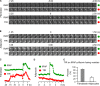
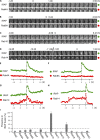
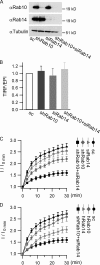
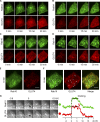
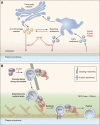
Rab proteins are important regulators of insulin-stimulated GLUT4 translocation to the plasma membrane (PM), but the precise steps in GLUT4 trafficking modulated by particular Rab proteins remain unclear. Here, we systematically investigate the involvement of Rab proteins in GLUT4 trafficking, focusing on Rab proteins directly mediating GLUT4 storage vesicle (GSV) delivery to the PM. Using dual-color total internal reflection fluorescence (TIRF) microscopy and an insulin-responsive aminopeptidase (IRAP)-pHluorin fusion assay, we demonstrated that Rab10 directly facilitated GSV translocation to and docking at the PM. Rab14 mediated GLUT4 delivery to the PM via endosomal compartments containing transferrin receptor (TfR), whereas Rab4A, Rab4B, and Rab8A recycled GLUT4 through the endosomal system. Myosin-Va associated with GSVs by interacting with Rab10, positioning peripherally recruited GSVs for ultimate fusion. Thus, multiple Rab proteins regulate the trafficking of GLUT4, with Rab10 coordinating with myosin-Va to mediate the final steps of insulin-stimulated GSV translocation to the PM.
Figures



References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

