Wnt-11 and Fz7 reduce cell adhesion in convergent extension by sequestration of PAPC and C-cadherin
- PMID: 22908314
- PMCID: PMC3514027
- DOI: 10.1083/jcb.201110076
Wnt-11 and Fz7 reduce cell adhesion in convergent extension by sequestration of PAPC and C-cadherin
Abstract
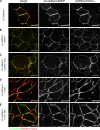
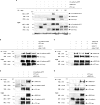
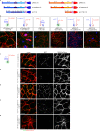
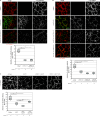
Wnt-11/planar cell polarity signaling polarizes mesodermal cells undergoing convergent extension during Xenopus laevis gastrulation. These shape changes associated with lateral intercalation behavior require a dynamic modulation of cell adhesion. In this paper, we report that Wnt-11/frizzled-7 (Fz7) controls cell adhesion by forming separate adhesion-modulating complexes (AMCs) with the paraxial protocadherin (PAPC; denoted as AMCP) and C-cadherin (denoted as AMCC) via distinct Fz7 interaction domains. When PAPC was part of a Wnt-11-Fz7 complex, its Dynamin1- and clathrin-dependent internalization was blocked. This membrane stabilization of AMCP (Fz7/PAPC) by Wnt-11 prevented C-cadherin clustering, resulting in reduced cell adhesion and modified cell sorting activity. Importantly, Wnt-11 did not influence C-cadherin internalization; instead, it promoted the formation of AMCC (Fz7/Cadherin), which competed with cis-dimerization of C-cadherin. Because PAPC and C-cadherin did not directly interact and did not form a joint complex with Fz7, we suggest that Wnt-11 triggers the formation of two distinct complexes, AMCC and AMCP, that act in parallel to reduce cell adhesion by hampering lateral clustering of C-cadherin.
Figures





Similar articles
-
PAPC and the Wnt5a/Ror2 pathway control the invagination of the otic placode in Xenopus.BMC Dev Biol. 2011 Jun 10;11:36. doi: 10.1186/1471-213X-11-36. BMC Dev Biol. 2011. PMID: 21663658 Free PMC article.
-
Paraxial protocadherin mediates cell sorting and tissue morphogenesis by regulating C-cadherin adhesion activity.J Cell Biol. 2006 Jul 17;174(2):301-13. doi: 10.1083/jcb.200602062. J Cell Biol. 2006. PMID: 16847104 Free PMC article.
-
Secreted Frizzled-related Protein 2 (sFRP2) Redirects Non-canonical Wnt Signaling from Fz7 to Ror2 during Vertebrate Gastrulation.J Biol Chem. 2016 Jun 24;291(26):13730-42. doi: 10.1074/jbc.M116.733766. Epub 2016 Apr 29. J Biol Chem. 2016. PMID: 27129770 Free PMC article.
-
Forces driving cell sorting in the amphibian embryo.Mech Dev. 2017 Apr;144(Pt A):81-91. doi: 10.1016/j.mod.2016.09.003. Epub 2016 Sep 30. Mech Dev. 2017. PMID: 27697520 Review.
-
Cadherin function during Xenopus gastrulation.Subcell Biochem. 2012;60:301-20. doi: 10.1007/978-94-007-4186-7_13. Subcell Biochem. 2012. PMID: 22674077 Review.
Cited by
-
Mechanics of Anteroposterior Axis Formation in Vertebrates.Annu Rev Cell Dev Biol. 2019 Oct 6;35:259-283. doi: 10.1146/annurev-cellbio-100818-125436. Epub 2019 Aug 14. Annu Rev Cell Dev Biol. 2019. PMID: 31412208 Free PMC article. Review.
-
Tissue patterning and cellular mechanics.J Cell Biol. 2015 Oct 26;211(2):219-31. doi: 10.1083/jcb.201506106. J Cell Biol. 2015. PMID: 26504164 Free PMC article. Review.
-
δ-Protocadherins regulate neural progenitor cell division by antagonizing Ryk and Wnt/β-catenin signaling.iScience. 2021 Jul 31;24(8):102932. doi: 10.1016/j.isci.2021.102932. eCollection 2021 Aug 20. iScience. 2021. PMID: 34430817 Free PMC article.
-
Regulation of Wnt signaling by protocadherins.Semin Cell Dev Biol. 2017 Sep;69:158-171. doi: 10.1016/j.semcdb.2017.07.043. Epub 2017 Aug 1. Semin Cell Dev Biol. 2017. PMID: 28774578 Free PMC article. Review.
-
Dishevelled controls bulk cadherin dynamics and the stability of individual cadherin clusters during convergent extension.Mol Biol Cell. 2022 Dec 1;33(14):br26. doi: 10.1091/mbc.E22-06-0194. Epub 2022 Oct 12. Mol Biol Cell. 2022. PMID: 36222834 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

