Smad3 binding to the foxp3 enhancer is dispensable for the development of regulatory T cells with the exception of the gut
- PMID: 22908322
- PMCID: PMC3428940
- DOI: 10.1084/jem.20112646
Smad3 binding to the foxp3 enhancer is dispensable for the development of regulatory T cells with the exception of the gut
Abstract
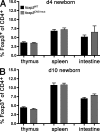
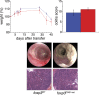
Regulatory T cells (T reg cells) are essential for the prevention of autoimmunity throughout life. T reg cell development occurs intrathymically but a subset of T reg cells can also differentiate from naive T cells in the periphery. In vitro, Smad signaling facilitates conversion of naive T cells into T reg cells but results in unstable Foxp3 expression. The TGF-β-Smad response element in the foxp3 locus is located in the CNS1 region in close proximity to binding sites for transcription factors implicated in TCR and retinoic acid signaling. From in vitro experiments it was previously postulated that foxp3 transcription represents a hierarchical process of transcription factor binding in which Smad3 would play a central role in transcription initiation. However, in vitro conditions generate T reg cells that differ from T reg cells encountered in vivo. To address the relevance of Smad3 binding to the CNS1 enhancer in vivo, we generated mice that exclusively lack the Smad binding site (foxp3(CNS1mut)). We show that binding of Smad3 to the foxp3 enhancer is dispensable for T reg cell development in newborn and adult mice with the exception of the gut.
Figures



Similar articles
-
Role of conserved non-coding DNA elements in the Foxp3 gene in regulatory T-cell fate.Nature. 2010 Feb 11;463(7282):808-12. doi: 10.1038/nature08750. Epub 2010 Jan 13. Nature. 2010. PMID: 20072126 Free PMC article.
-
Smad3 and NFAT cooperate to induce Foxp3 expression through its enhancer.Nat Immunol. 2008 Feb;9(2):194-202. doi: 10.1038/ni1549. Epub 2007 Dec 23. Nat Immunol. 2008. PMID: 18157133
-
Helminth secretions induce de novo T cell Foxp3 expression and regulatory function through the TGF-β pathway.J Exp Med. 2010 Oct 25;207(11):2331-41. doi: 10.1084/jem.20101074. Epub 2010 Sep 27. J Exp Med. 2010. PMID: 20876311 Free PMC article.
-
Induced Regulatory T Cells: Their Development, Stability, and Applications.Trends Immunol. 2016 Nov;37(11):803-811. doi: 10.1016/j.it.2016.08.012. Epub 2016 Sep 9. Trends Immunol. 2016. PMID: 27623114 Review.
-
Human regulatory T cells: a unique, stable thymic subset or a reversible peripheral state of differentiation?Immunol Lett. 2007 Nov 30;114(1):9-15. doi: 10.1016/j.imlet.2007.08.012. Epub 2007 Sep 29. Immunol Lett. 2007. PMID: 17945352 Free PMC article. Review.
Cited by
-
TGF-β signaling controls Foxp3 methylation and T reg cell differentiation by modulating Uhrf1 activity.J Exp Med. 2019 Dec 2;216(12):2819-2837. doi: 10.1084/jem.20190550. Epub 2019 Sep 12. J Exp Med. 2019. PMID: 31515281 Free PMC article.
-
Transcriptional control of regulatory T cell development and function.Trends Immunol. 2013 Nov;34(11):531-9. doi: 10.1016/j.it.2013.08.003. Epub 2013 Sep 6. Trends Immunol. 2013. PMID: 24016547 Free PMC article. Review.
-
Translating Treg Therapy for Inflammatory Bowel Disease in Humanized Mice.Cells. 2021 Jul 21;10(8):1847. doi: 10.3390/cells10081847. Cells. 2021. PMID: 34440615 Free PMC article. Review.
-
GARP: a surface molecule of regulatory T cells that is involved in the regulatory function and TGF-β releasing.Oncotarget. 2016 Jul 5;7(27):42826-42836. doi: 10.18632/oncotarget.8753. Oncotarget. 2016. PMID: 27095576 Free PMC article. Review.
-
The Active Form of Vitamin D Transcriptionally Represses Smad7 Signaling and Activates Extracellular Signal-regulated Kinase (ERK) to Inhibit the Differentiation of a Inflammatory T Helper Cell Subset and Suppress Experimental Autoimmune Encephalomyelitis.J Biol Chem. 2015 May 8;290(19):12222-36. doi: 10.1074/jbc.M114.621839. Epub 2015 Mar 25. J Biol Chem. 2015. PMID: 25809484 Free PMC article.
References
-
- Coombes J.L., Siddiqui K.R., Arancibia-Cárcamo C.V., Hall J., Sun C.M., Belkaid Y., Powrie F. 2007. A functionally specialized population of mucosal CD103+ DCs induces Foxp3+ regulatory T cells via a TGF-β and retinoic acid–dependent mechanism. J. Exp. Med. 204:1757–1764 10.1084/jem.20070590 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

