Implementing new health interventions in developing countries: why do we lose a decade or more?
- PMID: 22908877
- PMCID: PMC3495221
- DOI: 10.1186/1471-2458-12-683
Implementing new health interventions in developing countries: why do we lose a decade or more?
Abstract
Background: It is unclear how long it takes for health interventions to transition from research and development (R&D) to being used against diseases prevalent in resource-poor countries. We undertook an analysis of the time required to begin implementation of four vaccines and three malaria interventions. We evaluated five milestones for each intervention, and assessed if the milestones were associated with beginning implementation.
Methods: The authors screened World Health Organization (WHO) databases to determine the number of years between first regulatory approval of interventions, and countries beginning implementation. Descriptive analyses of temporal patterns and statistical analyses using logistic regression and Cox proportional hazard models were used to evaluate associations between five milestones and the beginning of implementation for each intervention. The milestones were: (A) presence of a coordinating group focused on the intervention; (B) availability of an intervention tailored to developing country health systems; (C) international financing commitment, and; (D) initial and (E) comprehensive WHO recommendations. Countries were categorized by World Bank income criteria.
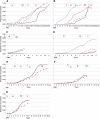
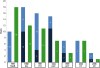
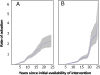
Results: Five years after regulatory approval, no low-income countries (LICs) had begun implementing any of the vaccines, increasing to an average of only 4% of LICs after 10 years. Each malaria intervention was used by an average of 7% of LICs after five years and 37% after 10 years. Four of the interventions had similar implementation rates to hepatitis B vaccine (HepB), while one was slower and one was faster than HepB. A financing commitment and initial WHO recommendation appeared to be temporally associated with the beginning of implementation. The initial recommendation from WHO was the only milestone associated in all statistical analyses with countries beginning implementation (relative rate = 1.97, P < 0.001).
Conclusions: Although possible that four milestones were not associated with countries beginning implementation, we propose an alternative interpretation; that the milestones were not realized early enough in each intervention's development to shorten the time to beginning implementation. We discuss a framework built upon existing literature for consideration during the development of future interventions. Identifying critical milestones and their timing relative to R&D, promises to help new interventions realize their intended public health impact more rapidly.
Figures


Similar articles
-
Global support for new vaccine implementation in middle-income countries.Vaccine. 2013 Apr 18;31 Suppl 2:B81-96. doi: 10.1016/j.vaccine.2012.11.085. Vaccine. 2013. PMID: 23598496 Review.
-
WHO policy development processes for a new vaccine: case study of malaria vaccines.Malar J. 2010 Jun 24;9:182. doi: 10.1186/1475-2875-9-182. Malar J. 2010. PMID: 20576114 Free PMC article.
-
Country planning for health interventions under development: lessons from the malaria vaccine decision-making framework and implications for other new interventions.Health Policy Plan. 2012 May;27 Suppl 2(Suppl 2):ii50-61. doi: 10.1093/heapol/czs039. Health Policy Plan. 2012. PMID: 22513733 Free PMC article.
-
The future of Cochrane Neonatal.Early Hum Dev. 2020 Nov;150:105191. doi: 10.1016/j.earlhumdev.2020.105191. Epub 2020 Sep 12. Early Hum Dev. 2020. PMID: 33036834
-
Conquering the intolerable burden of malaria: what's new, what's needed: a summary.Am J Trop Med Hyg. 2004 Aug;71(2 Suppl):1-15. Am J Trop Med Hyg. 2004. PMID: 15331814 Review.
Cited by
-
After 2015: infectious diseases in a new era of health and development.Philos Trans R Soc Lond B Biol Sci. 2014 May 12;369(1645):20130426. doi: 10.1098/rstb.2013.0426. Print 2014. Philos Trans R Soc Lond B Biol Sci. 2014. PMID: 24821913 Free PMC article. Review.
-
Assessing vaccine introduction and uptake timelines in Gavi-supported countries: are introduction timelines accelerating across vaccine delivery platforms?BMJ Glob Health. 2021 May;6(5):e005032. doi: 10.1136/bmjgh-2021-005032. BMJ Glob Health. 2021. PMID: 34045183 Free PMC article.
-
Assessing the operational feasibility and acceptability of an inhalable formulation of oxytocin for improving community-based prevention of postpartum haemorrhage in Myanmar: a qualitative inquiry.BMJ Open. 2018 Oct 24;8(10):e022140. doi: 10.1136/bmjopen-2018-022140. BMJ Open. 2018. PMID: 30361400 Free PMC article.
-
Transmission-Blocking Vaccines for Malaria: Time to Talk about Vaccine Introduction.Trends Parasitol. 2019 Jul;35(7):483-486. doi: 10.1016/j.pt.2019.04.008. Epub 2019 May 29. Trends Parasitol. 2019. PMID: 31153722 Free PMC article. Review.
-
Acceptability and Feasibility of a Low-Cost Device for Gestational Age Assessment in a Low-Resource Setting: Qualitative Study.JMIR Hum Factors. 2022 Dec 27;9(4):e34823. doi: 10.2196/34823. JMIR Hum Factors. 2022. PMID: 36574278 Free PMC article.
References
-
- Moran M, Guzman J, Henderson K, Abela-Oversteegen L, Wu L, The G-FINDER Report 2010: Neglected disease research and development: Is the global financing crisis changing R&D? London: Policy Cures; 2011.
-
- World Health Organization. Prequalification. www.who.int/topics/prequalification/en.
-
- World Health Organization. WHO Pesticide Evaluation Scheme: “WHOPES”. www.who.int/whopes/en/. www.who.int/whopes/en/
-
- Brooks AD, Wells WA, McLean TD, Khanna R, Coghlan R, Mertenskoetter T. et al. Ensuring that Developing Countries have Access to New Healthcare Products: The Role of Product Development Partnerships. Innovation Strategy Today. 2010;3:1–5.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

