Effects of activation on functional aster formation, microtubule assembly, and blastocyst development of goat oocytes injected with round spermatids
- PMID: 22908906
- PMCID: PMC3459009
- DOI: 10.1089/cell.2012.0029
Effects of activation on functional aster formation, microtubule assembly, and blastocyst development of goat oocytes injected with round spermatids
Abstract
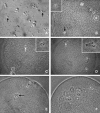
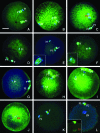
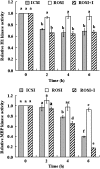
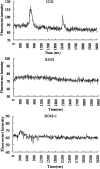
A systematic study was conducted on round spermatids (ROS) injection (ROSI) using the goat model. After ROSI, the oocytes were treated or not with ionomycin (ROSI+I and ROSI-I, respectively) and compared with intracytoplasmic sperm injection (ICSI). After ROSI-I, most oocytes were arrested with premature chromatin condensation and few oocytes formed pronuclei. In contrast, most oocytes formed pronuclei after ROSI+I. Some ROS were observed to form asters that organized a dense microtubule network after ROSI+I, but after ROSI-I, no ROS asters were observed. Whereas most of the oocytes showed Ca(2+) rises and a significant decline in maturation-promoting factor (MPF) and mitogen-activated protein kinase (MAPK) activities after ROSI+I, no such changes were observed after ROSI-I. Due to the lack of Ca(2+) oscillations after ROSI-I, oocytes were injected with more ROS. Interestingly, different from the results observed in a single ROS injection, injection with four ROS effectively activated oocytes by inducing typical Ca(2+) oscillations. Whereas ROSI+I oocytes and ICSI oocytes both showed extensive microtubule networks, no such a network was observed in parthenogenetic oocytes. Together, the results suggest that goat ROS is not able to trigger intracellular Ca(2+) rises and thus to inhibit MPF and MAPK activities, but artificial activation improved fertilization and development of ROSI goat oocytes. Goat ROS can organize functional microtubular asters in activated oocytes. A ROS-derived factor(s) may be essential for organization of a functional microtubule network to unite pronuclei. Goat centrosome is of paternal origin because both ROS and sperm asters organized an extensive microtubule network after intra-oocyte injection.
Figures
References
-
- Ajduk A. Małagocki A. Maleszewski M. Cytoplasmic maturation of mammalian oocytes: Development of a mechanism responsible for sperm-induced Ca2+ oscillations. Reprod. Biol. 2008;8:3–22. - PubMed
-
- Al-Hasani S. Ludwig M. Palermo I. Küpker W. Sandmann J. Johannisson R. Fornara P. Sturm R. Bals-Pratsch M. Bauer O. Diedrich K. Intracytoplasmic injection of round and elongated spermatids from azoospermic patients: results and review. Hum. Reprod. 1999;14(Suppl 1):97–107. - PubMed
-
- Balaban B. Urman B. Isiklar A. Alatas C. Aksoy S. Mercan R. Nuhoglu A. Progression to the blastocyst stage of embryos derived from testicular round spermatids. Hum. Reprod. 2000;15:1377–1382. - PubMed
-
- Chang H.Y. Levasseur M. Jones K.T. Degradation of APCcdc20 and APCcdh1 substrates during the second meiotic division in mouse eggs. J. Cell Sci. 2004;117(Pt 26):6289–6296. - PubMed
-
- Craft I. Bennett V. Nicholson N. Fertilizing ability of testicular spermatozoa. Lancet. 1993;342:864. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

