Coordinated Eph-ephrin signaling guides migration and axon targeting in the avian auditory system
- PMID: 22908944
- PMCID: PMC3515360
- DOI: 10.1186/1749-8104-7-29
Coordinated Eph-ephrin signaling guides migration and axon targeting in the avian auditory system
Abstract
Background: In the avian sound localization circuit, nucleus magnocellularis (NM) projects bilaterally to nucleus laminaris (NL), with ipsilateral and contralateral NM axon branches directed to dorsal and ventral NL dendrites, respectively. We previously showed that the Eph receptor EphB2 is expressed in NL neuropil and NM axons during development. Here we tested whether EphB2 contributes to NM-NL circuit formation.
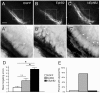
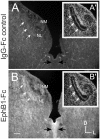
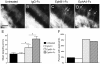
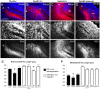
Results: We found that misexpression of EphB2 in embryonic NM precursors significantly increased the number of axon targeting errors from NM to contralateral NL in a cell-autonomous manner when forward signaling was impaired. We also tested the effects of inhibiting forward signaling of different Eph receptor subclasses by injecting soluble unclustered Fc-fusion proteins at stages when NM axons are approaching their NL target. Again we found an increase in axon targeting errors compared to controls when forward signaling was impaired, an effect that was significantly increased when both Eph receptor subclasses were inhibited together. In addition to axon targeting errors, we also observed morphological abnormalities of the auditory nuclei when EphB2 forward signaling was increased by E2 transfection, and when Eph-ephrin forward signaling was inhibited by E6-E8 injection of Eph receptor fusion proteins.
Conclusions: These data suggest that EphB signaling has distinct functions in axon guidance and morphogenesis. The results provide evidence that multiple Eph receptors work synergistically in the formation of precise auditory circuitry.
Figures



References
-
- Joseph AW, Hyson RL. Coincidence detection by binaural neurons in the chick brain stem. J Neurophysiol. 1993;69:1197–1211. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

