Site-resolved measurement of microsecond-to-millisecond conformational-exchange processes in proteins by solid-state NMR spectroscopy
- PMID: 22908968
- PMCID: PMC3557925
- DOI: 10.1021/ja303591y
Site-resolved measurement of microsecond-to-millisecond conformational-exchange processes in proteins by solid-state NMR spectroscopy
Abstract
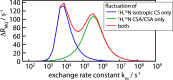
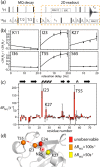
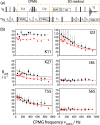
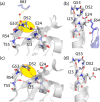
We demonstrate that conformational exchange processes in proteins on microsecond-to-millisecond time scales can be detected and quantified by solid-state NMR spectroscopy. We show two independent approaches that measure the effect of conformational exchange on transverse relaxation parameters, namely Carr-Purcell-Meiboom-Gill relaxation-dispersion experiments and measurement of differential multiple-quantum coherence decay. Long coherence lifetimes, as required for these experiments, are achieved by the use of highly deuterated samples and fast magic-angle spinning. The usefulness of the approaches is demonstrated by application to microcrystalline ubiquitin. We detect a conformational exchange process in a region of the protein for which dynamics have also been observed in solution. Interestingly, quantitative analysis of the data reveals that the exchange process is more than 1 order of magnitude slower than in solution, and this points to the impact of the crystalline environment on free energy barriers.
Figures
Similar articles
-
Probing microsecond time scale dynamics in proteins by methyl (1)H Carr-Purcell-Meiboom-Gill relaxation dispersion NMR measurements. Application to activation of the signaling protein NtrC(r).J Am Chem Soc. 2010 Dec 1;132(47):17004-14. doi: 10.1021/ja107410x. Epub 2010 Nov 8. J Am Chem Soc. 2010. PMID: 21058670 Free PMC article.
-
Microsecond time-scale conformational exchange in proteins: using long molecular dynamics trajectory to simulate NMR relaxation dispersion data.J Am Chem Soc. 2012 Feb 8;134(5):2555-62. doi: 10.1021/ja206442c. Epub 2012 Jan 27. J Am Chem Soc. 2012. PMID: 22206299
-
Microsecond Protein Dynamics from Combined Bloch-McConnell and Near-Rotary-Resonance R1p Relaxation-Dispersion MAS NMR.Chemphyschem. 2019 Jan 21;20(2):276-284. doi: 10.1002/cphc.201800935. Epub 2018 Dec 20. Chemphyschem. 2019. PMID: 30444575 Free PMC article.
-
Recent developments in deuterium solid-state NMR for the detection of slow motions in proteins.Solid State Nucl Magn Reson. 2021 Feb;111:101710. doi: 10.1016/j.ssnmr.2020.101710. Epub 2021 Jan 7. Solid State Nucl Magn Reson. 2021. PMID: 33450712 Free PMC article. Review.
-
Relaxation dispersion NMR spectroscopy for the study of protein allostery.Biophys Rev. 2015 Jun;7(2):191-200. doi: 10.1007/s12551-015-0166-6. Epub 2015 Feb 21. Biophys Rev. 2015. PMID: 28510170 Free PMC article. Review.
Cited by
-
Protein conformational dynamics studied by 15N and 1H R1ρ relaxation dispersion: Application to wild-type and G53A ubiquitin crystals.Solid State Nucl Magn Reson. 2017 Oct;87:86-95. doi: 10.1016/j.ssnmr.2017.04.002. Epub 2017 Apr 14. Solid State Nucl Magn Reson. 2017. PMID: 28438365 Free PMC article.
-
Effect of Post-Translational Modifications and Mutations on Amyloid-β Fibrils Dynamics at N Terminus.Biophys J. 2019 Oct 15;117(8):1524-1535. doi: 10.1016/j.bpj.2019.09.004. Epub 2019 Sep 12. Biophys J. 2019. PMID: 31570231 Free PMC article.
-
Amplitudes and time scales of picosecond-to-microsecond motion in proteins studied by solid-state NMR: a critical evaluation of experimental approaches and application to crystalline ubiquitin.J Biomol NMR. 2013 Nov;57(3):263-80. doi: 10.1007/s10858-013-9787-x. Epub 2013 Oct 9. J Biomol NMR. 2013. PMID: 24105432 Free PMC article.
-
Studying Dynamics by Magic-Angle Spinning Solid-State NMR Spectroscopy: Principles and Applications to Biomolecules.Prog Nucl Magn Reson Spectrosc. 2016 Aug;96:1-46. doi: 10.1016/j.pnmrs.2016.02.001. Epub 2016 Feb 15. Prog Nucl Magn Reson Spectrosc. 2016. PMID: 27110043 Free PMC article. Review.
-
Slow conformational exchange and overall rocking motion in ubiquitin protein crystals.Nat Commun. 2017 Jul 27;8(1):145. doi: 10.1038/s41467-017-00165-8. Nat Commun. 2017. PMID: 28747759 Free PMC article.
References
-
- Brüschweiler S.; Schanda P.; Kloiber K.; Brutscher B.; Kontaxis G.; Konrat R.; Tollinger M. J. Am. Chem. Soc. 2009, 131, 3063–3068. - PubMed
-
- Henzler-Wildman K.; Thai V.; Lei M.; Ott M.; Wolf-Watz M.; Fenn T.; Pozharski E.; Wilson M.; Petsko G.; Karplus M.; Hubner C.; Kern D. Nature 2007, 450, 838–U13. - PubMed
-
- Tzeng S.; Kalodimos C. Nature 2009, 462, 368–U139. - PubMed
-
- Boehr D.; McElheny D.; Dyson H.; Wright P. Science 2006, 313, 1638–1642. - PubMed
-
- Palmer A. Chem. Rev. 2004, 104, 3623–3640. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

