Site-resolved measurement of microsecond-to-millisecond conformational-exchange processes in proteins by solid-state NMR spectroscopy
- PMID: 22908968
- PMCID: PMC3557925
- DOI: 10.1021/ja303591y
Site-resolved measurement of microsecond-to-millisecond conformational-exchange processes in proteins by solid-state NMR spectroscopy
Abstract
We demonstrate that conformational exchange processes in proteins on microsecond-to-millisecond time scales can be detected and quantified by solid-state NMR spectroscopy. We show two independent approaches that measure the effect of conformational exchange on transverse relaxation parameters, namely Carr-Purcell-Meiboom-Gill relaxation-dispersion experiments and measurement of differential multiple-quantum coherence decay. Long coherence lifetimes, as required for these experiments, are achieved by the use of highly deuterated samples and fast magic-angle spinning. The usefulness of the approaches is demonstrated by application to microcrystalline ubiquitin. We detect a conformational exchange process in a region of the protein for which dynamics have also been observed in solution. Interestingly, quantitative analysis of the data reveals that the exchange process is more than 1 order of magnitude slower than in solution, and this points to the impact of the crystalline environment on free energy barriers.
Figures
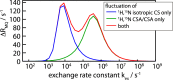
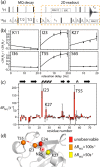
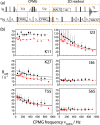
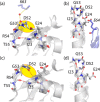
References
-
- Brüschweiler S.; Schanda P.; Kloiber K.; Brutscher B.; Kontaxis G.; Konrat R.; Tollinger M. J. Am. Chem. Soc. 2009, 131, 3063–3068. - PubMed
-
- Henzler-Wildman K.; Thai V.; Lei M.; Ott M.; Wolf-Watz M.; Fenn T.; Pozharski E.; Wilson M.; Petsko G.; Karplus M.; Hubner C.; Kern D. Nature 2007, 450, 838–U13. - PubMed
-
- Tzeng S.; Kalodimos C. Nature 2009, 462, 368–U139. - PubMed
-
- Boehr D.; McElheny D.; Dyson H.; Wright P. Science 2006, 313, 1638–1642. - PubMed
-
- Palmer A. Chem. Rev. 2004, 104, 3623–3640. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

