Lentiviral gene therapy against human immunodeficiency virus type 1, using a novel human TRIM21-cyclophilin A restriction factor
- PMID: 22909012
- PMCID: PMC3498886
- DOI: 10.1089/hum.2012.083
Lentiviral gene therapy against human immunodeficiency virus type 1, using a novel human TRIM21-cyclophilin A restriction factor
Abstract
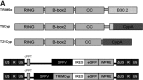
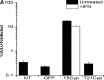
TRIM5α (tripartite motif-containing protein-5, isoform α)-cyclophilin A fusion proteins are anti-human immunodeficiency virus (HIV) restriction factors that have evolved in certain nonhuman primates over millions of years and protect against HIV and related viruses. Restriction by TRIM5αCypA is potent and highly resistant to viral escape by mutation and, in combination with a suitable gene delivery platform, offers the possibility of novel therapeutic approaches against HIV. Here we report that lentiviral vector delivery of human mimics of TRIM5α-cyclophilin A (TRIM5CypA) fusion proteins afforded robust and durable protection against HIV-1, but resulted in downregulation of host cell antiviral responses mediated by endogenous TRIM5α. We found that substitution of TRIM5α RING, B-box, and coiled-coil domains with similar domains from a related TRIM protein, TRIM21, produced a novel and equally potent inhibitor of HIV-1. Both TRIM5CypA and TRIM21CypA inhibited transduction by HIV-1-derived viral vectors and prevented propagation of replication-competent HIV-1 in human cell lines and in primary human T cells. Restriction factor-modified T cells exhibited preferential survival in the presence of wild-type HIV. Restriction was dependent on proteasomal degradation and was reversed in the presence of the cyclophilin inhibitor cyclosporin. Importantly, TRIM21CypA did not disturb endogenous TRIM5α-mediated restriction of gammaretroviral infection. Furthermore, endogenous TRIM21 antiviral activity was assessed by measuring inhibition of adenovirus-antibody complexes and was found to be preserved in all TRIMCypA-modified groups. We conclude that lentivirus-mediated expression of the novel chimeric restriction factor TRIM21CypA provides highly potent protection against HIV-1 without loss of normal innate immune TRIM activity.
Figures










References
-
- Demaison C. Parsley K. Brouns G., et al. High-level transduction and gene expression in hematopoietic repopulating cells using a human imunodeficiency virus type 1-based lentiviral vector containing an internal spleen focus forming virus promoter. Hum. Gene Ther. 2002;13:803–813. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

