Antitumor effect of new multiple antigen peptide based on HLA-A0201-restricted CTL epitopes of human telomerase reverse transcriptase (hTERT)
- PMID: 22909416
- PMCID: PMC7659284
- DOI: 10.1111/j.1349-7006.2012.02410.x
Antitumor effect of new multiple antigen peptide based on HLA-A0201-restricted CTL epitopes of human telomerase reverse transcriptase (hTERT)
Abstract
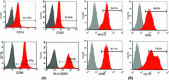
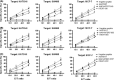
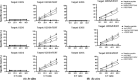
The development of peptide vaccines aimed at enhancing immune responses against tumor cells is becoming a promising area of research. Human telomerase reverse transcriptase (hTERT) is an ideal universal target for novel immunotherapies against cancers. The aim of this work was to verify whether the multiple antigen peptides (MAP) based on HLA-A0201-restricted CTL epitopes of hTERT could trigger a better and more sustained CTL response and kill multiple types of hTERT-positive tumor cells in vitro and ex vivo. Dendritic cells (DC) pulsed with MAP based on HLA-A0201-restricted CTL epitopes of hTERT (hTERT-540, hTERT-865 and hTERT-572Y) were used to evaluate immune responses against various tumors and were compared to the immune responses resulting from the use of corresponding linear epitopes and a recombinant adenovirus-hTERT vector. A 4-h standard (51) Cr-release assay and an ELISPOT assay were used for both in vitro and ex vivo analyses. Results demonstrated that targeting hTERT with an adenovector was the most effective way to stimulate a CD8(+) T cell response. When compared with linear hTERT epitopes, MAP could trigger stronger hTERT-specific CTL responses against tumor cells expressing hTERT and HLA-A0201. In contrast, the activated CTL could neither kill the hTERT-negative tumor cells, such as U2OS cells, nor kill HLA-A0201 negative cells, such as HepG2 cells. We also found that these peptide-specific CTL could not kill autologous lymphocytes and DC with low telomerase activity. Our results indicate that MAP from hTERT can be exploited for cancer immunotherapy.
© 2012 Japanese Cancer Association.
Figures





Similar articles
-
Diepitope multiple antigen peptide of hTERT trigger stronger anti-tumor immune responses in vitro.Int Immunopharmacol. 2013 Aug;16(4):444-50. doi: 10.1016/j.intimp.2013.05.006. Epub 2013 May 25. Int Immunopharmacol. 2013. PMID: 23714071
-
Immunogenic HLA-B*0702-restricted epitopes derived from human telomerase reverse transcriptase that elicit antitumor cytotoxic T-cell responses.Clin Cancer Res. 2006 May 15;12(10):3158-67. doi: 10.1158/1078-0432.CCR-05-2647. Clin Cancer Res. 2006. PMID: 16707616
-
Immunogenicity of a recombinant lentiviral vector carrying human telomerase tumor antigen in HLA-B*0702 transgenic mice.Vaccine. 2010 Aug 31;28(38):6374-81. doi: 10.1016/j.vaccine.2010.06.071. Epub 2010 Jul 21. Vaccine. 2010. PMID: 20654669
-
The immunogenicity of the hTERT540-548 peptide in cancer.Clin Cancer Res. 2008 Jan 1;14(1):4-7. doi: 10.1158/1078-0432.CCR-07-4590. Clin Cancer Res. 2008. PMID: 18172245 Review.
-
Prospects and challenges of building a cancer vaccine targeting telomerase.Biochimie. 2008 Jan;90(1):173-80. doi: 10.1016/j.biochi.2007.07.005. Epub 2007 Jul 17. Biochimie. 2008. PMID: 17716803 Free PMC article. Review.
Cited by
-
Novel metronomic chemotherapy and cancer vaccine combinatorial strategy for hepatocellular carcinoma in a mouse model.Cancer Immunol Immunother. 2015 Oct;64(10):1305-14. doi: 10.1007/s00262-015-1698-0. Epub 2015 May 6. Cancer Immunol Immunother. 2015. PMID: 25944003 Free PMC article.
-
Highly optimized DNA vaccine targeting human telomerase reverse transcriptase stimulates potent antitumor immunity.Cancer Immunol Res. 2013 Sep;1(3):179-189. doi: 10.1158/2326-6066.CIR-13-0001. Epub 2013 Jul 17. Cancer Immunol Res. 2013. PMID: 24777680 Free PMC article.
-
TERT promoter mutations are a major indicator of poor outcome in differentiated thyroid carcinomas.J Clin Endocrinol Metab. 2014 May;99(5):E754-65. doi: 10.1210/jc.2013-3734. Epub 2014 Jan 29. J Clin Endocrinol Metab. 2014. PMID: 24476079 Free PMC article.
-
Inhibition of tumor growth and metastasis by EMMPRIN multiple antigenic peptide (MAP) vaccination is mediated by immune modulation.Oncoimmunology. 2016 Nov 29;6(1):e1261778. doi: 10.1080/2162402X.2016.1261778. eCollection 2017. Oncoimmunology. 2016. PMID: 28197388 Free PMC article.
-
Gastric cancer vaccines synthesized using a TLR7 agonist and their synergistic antitumor effects with 5-fluorouracil.J Transl Med. 2018 May 8;16(1):120. doi: 10.1186/s12967-018-1501-z. J Transl Med. 2018. PMID: 29739434 Free PMC article.
References
-
- Stewart SA. Telomere maintenance and tumorigenesis. An “ALT” ernative road. Curr Mol Med 2005; 5: 253–7. - PubMed
-
- Janknecht R. On the road to immortality: hTERT upregulation in cancer cells. FEBS Lett 2004; 564: 9–13. - PubMed
-
- Jager E, Ringhoffer M, Karbach J, Arand M, Oesch F, Knuth A. Inverse relationship of melanocyte differentiation antigen expression in melanoma tissues and CD8+ cytotoxic‐T‐cell responses: evidence for immunoselection of antigen‐loss variants in vivo. Int J Cancer 1996; 66: 470–6. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

