Vascular density analysis in colorectal cancer patients treated with vatalanib (PTK787/ZK222584) in the randomised CONFIRM trials
- PMID: 22910317
- PMCID: PMC3461163
- DOI: 10.1038/bjc.2012.369
Vascular density analysis in colorectal cancer patients treated with vatalanib (PTK787/ZK222584) in the randomised CONFIRM trials
Abstract
Background: Pharmacological inhibitors of vascular endothelial growth factor (VEGF) receptors, like vatalanib, have been tested in randomised trials (CONFIRM (Colorectal Oral Novel therapy For the Inhibition of Angiogenesis and Retarding of Metastases) 1 and 2) in colorectal cancer showing activity in a subgroup of patients with high serum LDH expression. In the current study, we assessed the predictive role of vascular density (VD) in patients treated in the above trials.
Methods: Paraffin-embedded materials from 141 patients were analysed with immunohistochemistry for the expression of the CD31 (pan-endothelial cell marker) and of phosphorylated pVEGFR2/KDR on endothelial cells. The VD was correlated with response to therapy and with progression-free (PFS) and overall survival (OS).
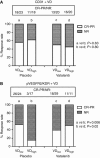
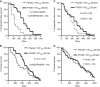
Results: A significant association of pVEGFR2/KDR+ VD with poor response in the placebo group was noted (response rates (RRs) 15% (3/20) when high VD vs 52% (26/50) when low VD; P=0.006). The RR increased from 15 (3/20) to 50% (11/22) in tumours with high VD when vatalanib was added to chemotherapy (P=0.02). A significantly improved PFS was noted in patients with high pVEGFR2/KDR+ VD when treated with vatalanib (P=0.002). A similar effect was also noted in patients with high CD31+ VD (P=0.07). Overall survival was marginally improved (P=0.07).
Conclusion: Assessment of the activated vessel density may allow the stratification of patients recruited in randomised trials with VEGFR-targeting anti-angiogenic agents, unmasking their therapeutic potential and enabling their introduction in the clinical practice for the benefit of specific patient subgroups, at the same time reducing the cost of therapy.
Conflict of interest statement
Author TT – Consultant or Advisory Role; Schering. Author GF – Honoraria; Novartis. Authors MMS and DL – Employment or Leadership Position; Novartis. Author TJ – Employment or Leadership Position; Bayer Oy. Author DL – Employment or Leadership Position; Bayer Pharma AG. Author GM – Employment or Leadership Position; Bayer Pharmaceuticals. The remaining authors declare no conflict of interest.
Figures

References
-
- Arapandoni-Dadioti P, Giatromanolaki A, Trihia H, Harris AL, Koukourakis MI (1999) Angiogenesis in ductal breast carcinoma. Comparison of microvessel density between primary tumour and lymph node metastasis. Cancer Lett 137: 145–150 - PubMed
-
- Brekken RA, Huang X, King SW, Thorpe PE (1998) Vascular endothelial growth factor as a marker of tumor endothelium. Cancer Res 58: 1952–1959 - PubMed
-
- Ferrara N, Davis-Smyth T (1997) The biology of vascular endothelial growth factor. Endocr Rev 18: 4–25 - PubMed
-
- Giatromanolaki A, Koukourakis MI, Sivridis E, Chlouverakis G, Vourvouhaki E, Turley H, Harris AL, Gatter KC (2007) Activated VEGFR2/KDR pathway in tumour cells and tumour associated vessels of colorectal cancer. Eur J Clin Invest 37: 878–886 - PubMed
-
- Giatromanolaki A, Koukourakis MI, Sivridis E, O’Byrne K, Gatter KC, Harris AL (2000) ‘Invading edge vs. inner’ (edvin) patterns of vascularization: an interplay between angiogenic and vascular survival factors defines the clinical behaviour of non-small cell lung cancer. J Pathol 192: 140–149 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

