Titin-based tension in the cardiac sarcomere: molecular origin and physiological adaptations
- PMID: 22910434
- PMCID: PMC3484226
- DOI: 10.1016/j.pbiomolbio.2012.08.003
Titin-based tension in the cardiac sarcomere: molecular origin and physiological adaptations
Abstract
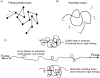
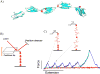
The passive stiffness of cardiac muscle plays a critical role in ventricular filling during diastole and is determined by the extracellular matrix and the sarcomeric protein titin. Titin spans from the Z-disk to the M-band of the sarcomere and also contains a large extensible region that acts as a molecular spring and develops passive force during sarcomere stretch. This extensible segment is titin's I-band region, and its force-generating mechanical properties determine titin-based passive tension. The properties of titin's I-band region can be modulated by isoform splicing and post-translational modification and are intimately linked to diastolic function. This review discusses the physical origin of titin-based passive tension, the mechanisms that alter titin stiffness, and titin's role in stress-sensing signaling pathways.
Copyright © 2012 Elsevier Ltd. All rights reserved.
Figures




References
-
- Anderson B, Bogomolovas J, et al. preparation
-
- Arber S, Halder G, et al. Muscle LIM protein, a novel essential regulator of myogenesis, promotes myogenic differentiation. Cell. 1994;79(2):221–231. - PubMed
-
- Arber S, Hunter JJ, et al. MLP-deficient mice exhibit a disruption of cardiac cytoarchitectural organization, dilated cardiomyopathy, and heart failure. Cell. 1997;88(3):393–403. - PubMed
-
- Bang ML, Centner T, et al. The complete gene sequence of titin, expression of an unusual approximately 700-kDa titin isoform, and its interaction with obscurin identify a novel Z-line to I-band linking system. Circ Res. 2001;89(11):1065–1072. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

