A meta-analysis of factors impacting detection of antidepressant efficacy in clinical trials: the importance of academic sites
- PMID: 22910458
- PMCID: PMC3499718
- DOI: 10.1038/npp.2012.153
A meta-analysis of factors impacting detection of antidepressant efficacy in clinical trials: the importance of academic sites
Abstract
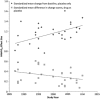
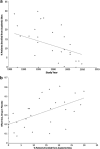
Variability in placebo response greatly complicates the design, conduct, and interpretation of clinical trials of antidepressant medications. To identify factors that impact detection of antidepressant-placebo differences, we conducted a meta-analysis of all relevant phase II-IV clinical trials for major depressive disorder conducted by the manufacturer of venlafaxine and desvenlafaxine completed by March 2011. We examined 15 factors potentially relevant to trial outcomes, using the standardized mean difference on the Hamilton Rating Scale for Depression (HAM-D₁₇) score as the primary outcome. Thirty trials comprising 8933 patients were included. In univariate analyses, antidepressant efficacy (ie, drug vs placebo difference) was predicted most strongly (β=3.74, p=0.0002) by the proportion of patients in the trial enrolled from academic sites. Other factors predicting larger drug-placebo differences included lower participant completion rate, fewer post-baseline study visits, earlier year of study, and study drug (venlafaxine>desvenlafaxine). In multivariate meta-regression modeling, only the proportion of patients from academic sites maintained statistical significance as a predictor of drug-placebo separation for both HAM-D₁₇ continuous score change (β=2.24, p=0.034) and response rate (β=2.26, p=0.035). Including a higher proportion of academic sites may increase the ability to detect differences between active drug and placebo in clinical trials of major depressive disorder.
Figures

References
-
- American Psychiatric Work Group on Major Depressive Disorder 2010Practice guideline for the treatment of patients with major depressive disorder, 3rd EditionAvailable at http://www.psychiatryonline.org/content.aspx?bookid=28§ionid=1667485 .
-
- Fava M, Evins AE, Dorer DJ, Schoenfeld DA. The problem of the placebo response in clinical trials for psychiatric disorders: culprits, possible remedies, and a novel study design approach. Psychother Psychosom. 2003;72:115–127. - PubMed
-
- Frank JD, Frank JB. Persuasion and Healing: A Comparative Study of Psychotherapy. Johns Hopkins Press, Baltimore, MD; 1991.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

