Neuroimmunoendocrine regulation of the prion protein in neutrophils
- PMID: 22910907
- PMCID: PMC3471762
- DOI: 10.1074/jbc.M112.394924
Neuroimmunoendocrine regulation of the prion protein in neutrophils
Abstract
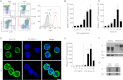
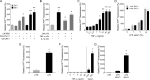
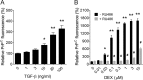
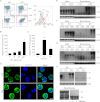
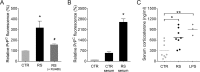
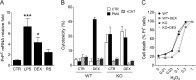
The prion protein (PrP(C)) is a cell surface protein expressed mainly in the nervous system. In addition to the role of its abnormal conformer in transmissible spongiform encephalopathies, normal PrP(C) may be implicated in other degenerative conditions often associated with inflammation. PrP(C) is also present in cells of hematopoietic origin, including T cells, dendritic cells, and macrophages, and it has been shown to modulate their functions. Here, we investigated the impact of inflammation and stress on the expression and function of PrP(C) in neutrophils, a cell type critically involved in both acute and chronic inflammation. We found that systemic injection of LPS induced transcription and translation of PrP(C) in mouse neutrophils. Up-regulation of PrP(C) was dependent on the serum content of TGF-β and glucocorticoids (GC), which, in turn, are contingent on the activation of the hypothalamic-pituitary-adrenal axis in response to systemic inflammation. GC and TGF-β, either alone or in combination, directly up-regulated PrP(C) in neutrophils, and accordingly, the blockade of GC receptors in vivo curtailed the LPS-induced increase in the content of PrP(C). Moreover, GC also mediated up-regulation of PrP(C) in neutrophils following noninflammatory restraint stress. Finally, neutrophils with up-regulated PrP(C) presented enhanced peroxide-dependent cytotoxicity to endothelial cells. The data demonstrate a novel interplay of the nervous, endocrine, and immune systems upon both the expression and function of PrP(C) in neutrophils, which may have a broad impact upon the physiology and pathology of various organs and systems.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

