Pregnancy-induced noncoding RNA (PINC) associates with polycomb repressive complex 2 and regulates mammary epithelial differentiation
- PMID: 22911650
- PMCID: PMC3406180
- DOI: 10.1371/journal.pgen.1002840
Pregnancy-induced noncoding RNA (PINC) associates with polycomb repressive complex 2 and regulates mammary epithelial differentiation
Abstract
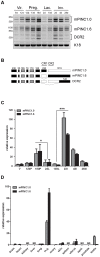
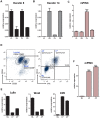
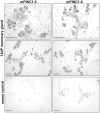
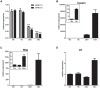
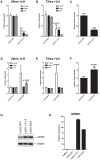
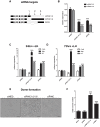
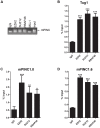
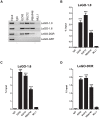
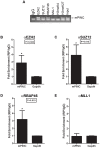
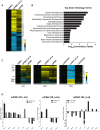
Pregnancy-induced noncoding RNA (PINC) and retinoblastoma-associated protein 46 (RbAp46) are upregulated in alveolar cells of the mammary gland during pregnancy and persist in alveolar cells that remain in the regressed lobules following involution. The cells that survive involution are thought to function as alveolar progenitor cells that rapidly differentiate into milk-producing cells in subsequent pregnancies, but it is unknown whether PINC and RbAp46 are involved in maintaining this progenitor population. Here, we show that, in the post-pubertal mouse mammary gland, mPINC is enriched in luminal and alveolar progenitors. mPINC levels increase throughout pregnancy and then decline in early lactation, when alveolar cells undergo terminal differentiation. Accordingly, mPINC expression is significantly decreased when HC11 mammary epithelial cells are induced to differentiate and produce milk proteins. This reduction in mPINC levels may be necessary for lactation, as overexpression of mPINC in HC11 cells blocks lactogenic differentiation, while knockdown of mPINC enhances differentiation. Finally, we demonstrate that mPINC interacts with RbAp46, as well as other members of the polycomb repressive complex 2 (PRC2), and identify potential targets of mPINC that are differentially expressed following modulation of mPINC expression levels. Taken together, our data suggest that mPINC inhibits terminal differentiation of alveolar cells during pregnancy to prevent abundant milk production and secretion until parturition. Additionally, a PRC2 complex that includes mPINC and RbAp46 may confer epigenetic modifications that maintain a population of mammary epithelial cells committed to the alveolar fate in the involuted gland.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures

Similar articles
-
Polycomb group gene Ezh2 regulates mammary gland morphogenesis and maintains the luminal progenitor pool.Stem Cells. 2013 Sep;31(9):1910-20. doi: 10.1002/stem.1437. Stem Cells. 2013. PMID: 23712803
-
Alveolar progenitor cells develop in mouse mammary glands independent of pregnancy and lactation.J Cell Physiol. 2007 Sep;212(3):729-36. doi: 10.1002/jcp.21071. J Cell Physiol. 2007. PMID: 17443685
-
Zinc Finger Homeodomain Factor Zfhx3 Is Essential for Mammary Lactogenic Differentiation by Maintaining Prolactin Signaling Activity.J Biol Chem. 2016 Jun 10;291(24):12809-12820. doi: 10.1074/jbc.M116.719377. Epub 2016 Apr 20. J Biol Chem. 2016. PMID: 27129249 Free PMC article.
-
Remodeling of Murine Mammary Adipose Tissue during Pregnancy, Lactation, and Involution.J Mammary Gland Biol Neoplasia. 2019 Sep;24(3):207-212. doi: 10.1007/s10911-019-09434-2. Epub 2019 Sep 12. J Mammary Gland Biol Neoplasia. 2019. PMID: 31512027 Free PMC article. Review.
-
Alveolar cells in the mammary gland: lineage commitment and cell death.Biochem J. 2022 May 13;479(9):995-1006. doi: 10.1042/BCJ20210734. Biochem J. 2022. PMID: 35551601 Free PMC article. Review.
Cited by
-
Long Intergenic Non-Coding RNAs in the Mammary Parenchyma and Fat Pad of Pre-Weaning Heifer Calves: Identification and Functional Analysis.Animals (Basel). 2021 Apr 28;11(5):1268. doi: 10.3390/ani11051268. Animals (Basel). 2021. PMID: 33924848 Free PMC article.
-
Behind the scenes: How RNA orchestrates the epigenetic regulation of gene expression.Front Cell Dev Biol. 2023 Jan 25;11:1123975. doi: 10.3389/fcell.2023.1123975. eCollection 2023. Front Cell Dev Biol. 2023. PMID: 36760365 Free PMC article. Review.
-
Interpreting the molecular mechanisms of RBBP4/7 and their roles in human diseases (Review).Int J Mol Med. 2024 May;53(5):48. doi: 10.3892/ijmm.2024.5372. Epub 2024 Apr 5. Int J Mol Med. 2024. PMID: 38577935 Free PMC article. Review.
-
New twists on long noncoding RNAs: from mobile elements to motile cancer cells.RNA Biol. 2020 Nov;17(11):1535-1549. doi: 10.1080/15476286.2020.1760535. Epub 2020 Jun 10. RNA Biol. 2020. PMID: 32522127 Free PMC article. Review.
-
Long noncoding RNAs and tumorigenesis: genetic associations, molecular mechanisms, and therapeutic strategies.Tumour Biol. 2016 Jan;37(1):163-75. doi: 10.1007/s13277-015-4445-4. Epub 2015 Nov 19. Tumour Biol. 2016. PMID: 26586396 Review.
References
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

