Targeting carcinoma-associated fibroblasts within the tumor stroma with a fibroblast activation protein-activated prodrug
- PMID: 22911669
- PMCID: PMC3529592
- DOI: 10.1093/jnci/djs336
Targeting carcinoma-associated fibroblasts within the tumor stroma with a fibroblast activation protein-activated prodrug
Abstract
Background: Fibroblasts undergo a morphological transformation to a reactive phenotype in the tumor microenvironment characterized by the expression of proteins such as fibroblast activation protein (FAP), a post-prolyl endopeptidase with expression largely restricted to carcinoma-associated fibroblasts. Thapsigargin (TG) is a highly toxic natural plant product that triggers a rise in intracellular calcium levels and apoptosis. FAP is therefore a provocative target for the activation of prodrugs consisting of a FAP-specific peptide coupled to a potent cytotoxic analog of TG.
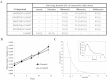
Methods: The efficacy of FAP-activated peptidyl-TG prodrugs was tested in vitro in cell proliferation assays and effects on intracellular calcium in human cancer cell lines. The effects of FAP-activated prodrugs on tumor growth and host toxicity were tested in Balb-C nude MCF-7 and LNCaP xenograft mice (n = 9-11 per group). P values were calculated using permutation tests based on 50 000 permutations. Mixed effects models were used to account for correlations among replicate measures. All statistical tests were two-sided.
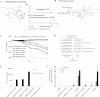
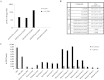
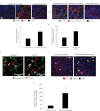
Results: FAP-activated prodrugs killed human cancer cells at low nanomolar concentrations (MCF-7 cells: IC(50) = 3.5 nM). Amino acid-12ADT analogs from FAP-cleaved prodrugs, but not uncleaved prodrugs, produced a rapid rise in intracellular calcium within minutes of exposure. Immunohistochemical analysis of xenografts exposed to FAP-prodrugs documented stromal-selective cell death of fibroblasts, pericytes, and endothelial cells of sufficient magnitude to inhibit growth of MCF-7 and LNCaP xenografts with minimal systemic toxicity, whereas non-FAP cleavable prodrugs were inactive. MCF-7 and LNCaP xenografts treated with a FAP-activated prodrug had maximal treated-to-control tumor volume ratios of 0.36 (treated: mean = 0.206 mm(3), 95% CI = 0.068 to 0.344 mm(3); control: mean = 0.580 mm(3), 95% CI = 0.267 to 0.893 mm(3)) and 0.24 (treated: mean = 0.131 mm(3), 95% CI = 0.09 to 0.180 mm(3); control: mean = 0.543 mm(3), 95% CI = 0.173 to 0.913 mm(3)), respectively, on day 21 after therapy.
Conclusions: This study validates the proteolytic activity of FAP as a target for the activation of a systemically delivered cytotoxic prodrug and demonstrates that targeted killing of cells within the stromal compartment of the tumor microenvironment can produce a therapeutic response.
Figures



Similar articles
-
Iterative design of emetine-based prodrug targeting fibroblast activation protein (FAP) and dipeptidyl peptidase IV DPPIV using a tandem enzymatic activation strategy.Prostate. 2016 Jun;76(8):703-14. doi: 10.1002/pros.23162. Epub 2016 Feb 2. Prostate. 2016. PMID: 26835873 Free PMC article.
-
Pharmacokinetics and toxicology of a fibroblast activation protein (FAP)-activated prodrug in murine xenograft models of human cancer.Prostate. 2014 Sep;74(13):1308-19. doi: 10.1002/pros.22847. Epub 2014 Jul 22. Prostate. 2014. PMID: 25053236 Free PMC article.
-
Targeting the cancer stroma with a fibroblast activation protein-activated promelittin protoxin.Mol Cancer Ther. 2009 May;8(5):1378-86. doi: 10.1158/1535-7163.MCT-08-1170. Epub 2009 May 5. Mol Cancer Ther. 2009. PMID: 19417147 Free PMC article.
-
Molecular recognition of fibroblast activation protein for diagnostic and therapeutic applications.Biochim Biophys Acta Proteins Proteom. 2020 Jul;1868(7):140409. doi: 10.1016/j.bbapap.2020.140409. Epub 2020 Apr 6. Biochim Biophys Acta Proteins Proteom. 2020. PMID: 32171757 Review.
-
Rationale behind targeting fibroblast activation protein-expressing carcinoma-associated fibroblasts as a novel chemotherapeutic strategy.Mol Cancer Ther. 2012 Feb;11(2):257-66. doi: 10.1158/1535-7163.MCT-11-0340. Mol Cancer Ther. 2012. PMID: 22323494 Free PMC article. Review.
Cited by
-
Can Targeting Stroma Pave the Way to Enhanced Antitumor Immunity and Immunotherapy of Solid Tumors?Cancer Immunol Res. 2016 Apr;4(4):269-78. doi: 10.1158/2326-6066.CIR-16-0011. Cancer Immunol Res. 2016. PMID: 27036971 Free PMC article. Review.
-
The axis of tumor-associated macrophages, extracellular matrix proteins, and cancer-associated fibroblasts in oncogenesis.Cancer Cell Int. 2024 Oct 7;24(1):335. doi: 10.1186/s12935-024-03518-8. Cancer Cell Int. 2024. PMID: 39375726 Free PMC article. Review.
-
Role of Serine Proteases at the Tumor-Stroma Interface.Front Immunol. 2022 Feb 11;13:832418. doi: 10.3389/fimmu.2022.832418. eCollection 2022. Front Immunol. 2022. PMID: 35222418 Free PMC article. Review.
-
Concise Review: Mesenchymal Stem Cell-Based Drug Delivery: The Good, the Bad, the Ugly, and the Promise.Stem Cells Transl Med. 2018 Sep;7(9):651-663. doi: 10.1002/sctm.18-0024. Epub 2018 Aug 1. Stem Cells Transl Med. 2018. PMID: 30070053 Free PMC article. Review.
-
Heterotypic cellular interactions in the ovarian tumor microenvironment: biological significance and therapeutic implications.Front Oncol. 2014 Feb 6;4:18. doi: 10.3389/fonc.2014.00018. eCollection 2014. Front Oncol. 2014. PMID: 24567915 Free PMC article. Review.
References
-
- Bast R Jr Kufe DW Pollock RE Weichselbaum RR Holland JF Frei E III Gansler TS Cancer Medicine. 5thed. Hamilton, ON: Decker BC; 2000.
-
- Hewitt RE, Powe DG, Carter GI, Turner DR. Desmoplasia and its relevance to colorectal tumour invasion. Int J Cancer 1993;53(1):62–69 - PubMed
-
- Rønnov-Jessen L, Petersen OW, Bissell MJ. Cellular changes involved in conversion of normal to malignant breast: importance of the stromal reaction. Physiol Rev 1996;76(1):69–125 - PubMed
-
- Mueller MM, Fusenig NE. Friends or foes—bipolar effects of the tumour stroma in cancer. Nat Rev Cancer 2004;4(11):839–849 - PubMed
-
- Allinen M, Beroukhim R, Cai L, et al. Molecular characterization of the tumor microenvironment in breast cancer. Cancer Cell 2004;6(1):17–32 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

