Substrate stiffness and oxygen as regulators of stem cell differentiation during skeletal tissue regeneration: a mechanobiological model
- PMID: 22911707
- PMCID: PMC3404068
- DOI: 10.1371/journal.pone.0040737
Substrate stiffness and oxygen as regulators of stem cell differentiation during skeletal tissue regeneration: a mechanobiological model
Abstract
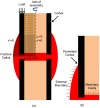
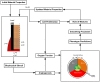
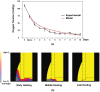
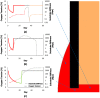
Extrinsic mechanical signals have been implicated as key regulators of mesenchymal stem cell (MSC) differentiation. It has been possible to test different hypotheses for mechano-regulated MSC differentiation by attempting to simulate regenerative events such as bone fracture repair, where repeatable spatial and temporal patterns of tissue differentiation occur. More recently, in vitro studies have identified other environmental cues such as substrate stiffness and oxygen tension as key regulators of MSC differentiation; however it remains unclear if and how such cues determine stem cell fate in vivo. As part of this study, a computational model was developed to test the hypothesis that substrate stiffness and oxygen tension regulate stem cell differentiation during fracture healing. Rather than assuming mechanical signals act directly on stem cells to determine their differentiation pathway, it is postulated that they act indirectly to regulate angiogenesis and hence partially determine the local oxygen environment within a regenerating tissue. Chondrogenesis of MSCs was hypothesized to occur in low oxygen regions, while in well vascularised regions of the regenerating tissue a soft local substrate was hypothesised to facilitate adipogenesis while a stiff substrate facilitated osteogenesis. Predictions from the model were compared to both experimental data and to predictions of a well established computational mechanobiological model where tissue differentiation is assumed to be regulated directly by the local mechanical environment. The model predicted all the major events of fracture repair, including cartilaginous bridging, endosteal and periosteal bony bridging and bone remodelling. It therefore provides support for the hypothesis that substrate stiffness and oxygen play a key role in regulating MSC fate during regenerative events such as fracture healing.
Conflict of interest statement
Figures


References
-
- Pauwels F. [A new theory on the influence of mechanical stimuli on the differentiation of supporting tissue. The tenth contribution to the functional anatomy and causal morphology of the supporting structure]. Z Anat Entwicklungsgesch. 1960;121:478–515. - PubMed
-
- Carter DR, Beaupre GS, Giori NJ, Helms JA. Mechanobiology of skeletal regeneration. Clin Orthop Relat Res. 1998. pp. S41–55. - PubMed
-
- Carter DR, Blenman PR, Beaupre GS. Correlations between mechanical stress history and tissue differentiation in initial fracture healing. J Orthop Res. 1988;6:736–748. - PubMed
-
- Claes LE, Heigele CA. Magnitudes of local stress and strain along bony surfaces predict the course and type of fracture healing. J Biomech. 1999;32:255–266. - PubMed
-
- Prendergast PJ, Huiskes R, Soballe K. ESB Research Award 1996. Biophysical stimuli on cells during tissue differentiation at implant interfaces. J Biomech. 1997;30:539–548. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Research Materials

