A Wnt/beta-catenin pathway antagonist Chibby binds Cenexin at the distal end of mother centrioles and functions in primary cilia formation
- PMID: 22911743
- PMCID: PMC3401179
- DOI: 10.1371/journal.pone.0041077
A Wnt/beta-catenin pathway antagonist Chibby binds Cenexin at the distal end of mother centrioles and functions in primary cilia formation
Abstract
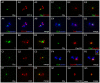
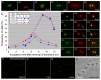
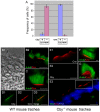
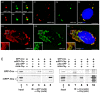
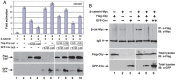
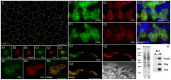
The mother centriole of the centrosome is distinguished from immature daughter centrioles by the presence of accessory structures (distal and subdistal appendages), which play an important role in the organization of the primary cilium in quiescent cells. Primary cilia serve as sensory organelles, thus have been implicated in mediating intracellular signal transduction pathways. Here we report that Chibby (Cby), a highly conserved antagonist of the Wnt/β-catenin pathway, is a centriolar component specifically located at the distal end of the mother centriole and essential for assembly of the primary cilium. Cby appeared as a discrete dot in the middle of a ring-like structure revealed by staining with a distal appendage component of Cep164. Cby interacted with one of the appendage components, Cenexin (Cnx), which thereby abrogated the inhibitory effect of Cby on β-catenin-mediated transcriptional activation in a dose-dependent manner. Cby and Cnx did not precisely align, as Cby was detected at a more distal position than Cnx. Cnx emerged earlier than Cby during the cell cycle and was required for recruitment of Cby to the mother centriole. However, Cby was dispensable for Cnx localization to the centriole. During massive centriogenesis in in vitro cultured mouse tracheal epithelial cells, Cby and Cnx were expressed in a similar pattern, which was coincident with the expression of Foxj1. Our results suggest that Cby plays an important role in organization of both primary and motile cilia in collaboration with Cnx.
Conflict of interest statement
Figures



References
-
- Kleylein-Sohn J, Westendorf J, Le Clech M, Habedanck R, Stierhof YD, et al. Plk4-induced centriole biogenesis in human cells. Dev. Cell. 2007;13:190–202. - PubMed
-
- Schmidt TI, Kleylein-Sohn J, Westendorf J, Le Clech M, Lavoie SB, et al. Control of centriole length by CPAP and CP110. Curr. Biol. 2009;19:1005–1011. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

