BLM and RMI1 alleviate RPA inhibition of TopoIIIα decatenase activity
- PMID: 22911760
- PMCID: PMC3401101
- DOI: 10.1371/journal.pone.0041208
BLM and RMI1 alleviate RPA inhibition of TopoIIIα decatenase activity
Abstract
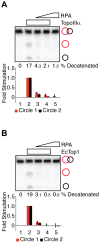
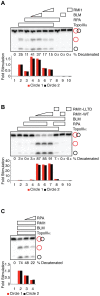
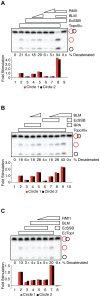
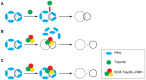
RPA is a single-stranded DNA binding protein that physically associates with the BLM complex. RPA stimulates BLM helicase activity as well as the double Holliday junction dissolution activity of the BLM-topoisomerase IIIα complex. We investigated the effect of RPA on the ssDNA decatenase activity of topoisomerase IIIα. We found that RPA and other ssDNA binding proteins inhibit decatenation by topoisomerase IIIα. Complex formation between BLM, TopoIIIα, and RMI1 ablates inhibition of decatenation by ssDNA binding proteins. Together, these data indicate that inhibition by RPA does not involve species-specific interactions between RPA and BLM-TopoIIIα-RMI1, which contrasts with RPA modulation of double Holliday junction dissolution. We propose that topoisomerase IIIα and RPA compete to bind to single-stranded regions of catenanes. Interactions with BLM and RMI1 enhance toposiomerase IIIα activity, promoting decatenation in the presence of RPA.
Conflict of interest statement
Figures

Similar articles
-
The dissolution of double Holliday junctions.Cold Spring Harb Perspect Biol. 2014 Jul 1;6(7):a016477. doi: 10.1101/cshperspect.a016477. Cold Spring Harb Perspect Biol. 2014. PMID: 24984776 Free PMC article. Review.
-
Role of replication protein A in double holliday junction dissolution mediated by the BLM-Topo IIIα-RMI1-RMI2 protein complex.J Biol Chem. 2013 May 17;288(20):14221-14227. doi: 10.1074/jbc.M113.465609. Epub 2013 Mar 30. J Biol Chem. 2013. PMID: 23543748 Free PMC article.
-
Human topoisomerase IIIalpha is a single-stranded DNA decatenase that is stimulated by BLM and RMI1.J Biol Chem. 2010 Jul 9;285(28):21426-36. doi: 10.1074/jbc.M110.123216. Epub 2010 May 5. J Biol Chem. 2010. PMID: 20445207 Free PMC article.
-
Multifaceted role of the Topo IIIα-RMI1-RMI2 complex and DNA2 in the BLM-dependent pathway of DNA break end resection.Nucleic Acids Res. 2014;42(17):11083-91. doi: 10.1093/nar/gku803. Epub 2014 Sep 8. Nucleic Acids Res. 2014. PMID: 25200081 Free PMC article.
-
A Structural Guide to the Bloom Syndrome Complex.Structure. 2021 Feb 4;29(2):99-113. doi: 10.1016/j.str.2020.11.020. Epub 2020 Dec 22. Structure. 2021. PMID: 33357470 Review.
Cited by
-
RADX condenses single-stranded DNA to antagonize RAD51 loading.Nucleic Acids Res. 2020 Aug 20;48(14):7834-7843. doi: 10.1093/nar/gkaa559. Nucleic Acids Res. 2020. PMID: 32621611 Free PMC article.
-
The dissolution of double Holliday junctions.Cold Spring Harb Perspect Biol. 2014 Jul 1;6(7):a016477. doi: 10.1101/cshperspect.a016477. Cold Spring Harb Perspect Biol. 2014. PMID: 24984776 Free PMC article. Review.
-
Unravelling the mechanisms of Type 1A topoisomerases using single-molecule approaches.Nucleic Acids Res. 2021 Jun 4;49(10):5470-5492. doi: 10.1093/nar/gkab239. Nucleic Acids Res. 2021. PMID: 33963870 Free PMC article. Review.
-
The many lives of type IA topoisomerases.J Biol Chem. 2020 May 15;295(20):7138-7153. doi: 10.1074/jbc.REV120.008286. Epub 2020 Apr 10. J Biol Chem. 2020. PMID: 32277049 Free PMC article. Review.
-
TopA, the Sulfolobus solfataricus topoisomerase III, is a decatenase.Nucleic Acids Res. 2018 Jan 25;46(2):861-872. doi: 10.1093/nar/gkx1247. Nucleic Acids Res. 2018. PMID: 29253195 Free PMC article.
References
-
- Hickson ID. RecQ helicases: caretakers of the genome. Nat Rev Cancer. 2003;3:169–178. - PubMed
-
- Ellis NA, Groden J, Ye TZ, Straughen J, Lennon DJ, et al. The Bloom's syndrome gene product is homologous to RecQ helicases. Cell. 1995;83:655–666. - PubMed
-
- Wu L, Hickson ID. The Bloom's syndrome helicase suppresses crossing over during homologous recombination. Nature. 2003;426:870–874. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

