Potential role for PAD2 in gene regulation in breast cancer cells
- PMID: 22911765
- PMCID: PMC3404060
- DOI: 10.1371/journal.pone.0041242
Potential role for PAD2 in gene regulation in breast cancer cells
Abstract
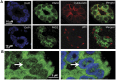
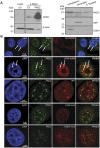
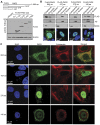
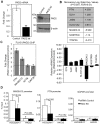
The peptidylarginine deiminase (PAD) family of enzymes post-translationally convert positively charged arginine residues in substrate proteins to the neutral, non-standard residue citrulline. PAD family members 1, 2, 3, and 6 have previously been localized to the cell cytoplasm and, thus, their potential to regulate gene activity has not been described. We recently demonstrated that PAD2 is expressed in the canine mammary gland epithelium and that levels of histone citrullination in this tissue correlate with PAD2 expression. Given these observations, we decided to test whether PAD2 might localize to the nuclear compartment of the human mammary epithelium and regulate gene activity in these cells. Here we show, for the first time, that PAD2 is specifically expressed in human mammary gland epithelial cells and that a portion of PAD2 associates with chromatin in MCF-7 breast cancer cells. We investigated a potential nuclear function for PAD2 by microarray, qPCR, and chromatin immunoprecipitation analysis. Results show that the expression of a unique subset of genes is disregulated following depletion of PAD2 from MCF-7 cells. Further, ChIP analysis of two of the most highly up- and down-regulated genes (PTN and MAGEA12, respectively) found that PAD2 binds directly to these gene promoters and that the likely mechanism by which PAD2 regulates expression of these genes is via citrullination of arginine residues 2-8-17 on histone H3 tails. Thus, our findings define a novel role for PAD2 in gene expression in human mammary epithelial cells.
Conflict of interest statement
Figures

References
-
- Suzuki A, Yamada R, Chang X, Tokuhiro S, Sawada T, et al. Functional haplotypes of PADI4, encoding citrullinating enzyme peptidylarginine deiminase 4, are associated with rheumatoid arthritis. Nat Genet. 2003;34:395–402. - PubMed
-
- Ishigami A, Ohsawa T, Hiratsuka M, Taguchi H, Kobayashi S, et al. Abnormal accumulation of citrullinated proteins catalyzed by peptidylarginine deiminase in hippocampal extracts from patients with Alzheimer’s disease. J Neurosci Res. 2005;80:120–128. - PubMed
-
- Chang X, Han J. Expression of peptidylarginine deiminase type 4 (PAD4) in various tumors. Mol Carcinog. 2006;45:183–196. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

